Environmental contamination in intensive care units (ICU) plays a very important role in the transmission of pathogens like Acinetobacter baumannii (A. baumannii). The healthcare professionals’ hand and glove contamination after contact with the patients and the environmental contamination is one of the mechanisms of usual transmission.1–3 Some studies have shown that patients hospitalized in a room previously occupied by a patient colonized/infected by multiresistant microorganisms (MRM) have a much higher risk of acquiring one of these pathogens than a patient hospitalized in a room whose prior occupant was not colonized/infected.1,4 The terminal decontamination that is usually conducted after a patient is discharged from the hospital is not enough. This has been proven in various studies that confirm that up to 50 per cent of the room surfaces cannot be adequately decontaminated.5 In order to reduce environmental contamination several methods of disinfection have been developed using hydrogen peroxide and ultraviolet light.6 Due to the existence of multiple machines and medical equipment, the process of decontaminating an ICU examination room is a hard task that is loaded with obstacles, and almost impossible to accomplish thoroughly in a short period of time. In high-volume centres—with permanent and urgent change of patients, carrying out this thorough decontamination process becomes nearly impossible, which gradually increases the bacterial load favouring the transmission of microorganisms. This added to the specific characteristics of critical patients, the difficulties trying to observe the strict/permanent hand washing protocols, and the lack of scrupulous observance in the isolation of colonized/infected patients, leads to the perfect storm that explains the elevated number of ICU nosocomial infections.
A. baumannii is a huge problem and a global healthcare challenge. It is one of the most common microorganisms responsible for the infections of critical patients and causes serious infections such as ventilator-associated pneumonias or bacteriemias.7,8 Some of its characteristics are its high adhesiveness; its capacity to survive in the environment; and the difficulties found when trying to eliminate it with the usual hygienic measures, which allows its easy colonization of surfaces and medical equipment. In studies conducted on epidemic outbreaks in ICUs, A. baumannii has been identified in reservoirs such as respirators, screens, humidifiers, pressure transducers, thermometers, or mattresses.7,9 These outbreaks may last in time and become endemic due to the large environmental colonization load, becoming a huge problem of great clinical/epidemiological repercussions that aggravates due to its usual multiresistance. The hospital fomites, the colonizations/infections of patients, and the healthcare providers make up an interrelated triangle that feeds back and forth perpetuating the transmission of this microorganism and maintaining the endemic situation, which is why the strict observance of hand hygiene by the healthcare personnel is always essential if we want to control an endemic situation.
Our hospital polyvalent ICU experienced one multiresistant A. baumannii endemia for eighteen years. During all this time, the classical measures recommended by the medical literature were implemented: cultures for epidemiological monitoring; isolation of colonized/infected patients; when possible, isolation of colonized/infected patient cohorts in a specific unit; programmes of annual thorough environmental decontamination (included new paint on the surfaces); hand hygiene; and personnel training (Table 1). All these measures proved ineffective and the endemia was even more serious after we moved to a new hospital, indicative that a new architectonic environment free from MRM that, a priori, is a favourable scenario does not solve a situation of endemia if we implement the same measures. The colonized/infected patients and the contaminated medical equipment were transferred to the new hospital, but the historic endemic situation was maintained since this transfer was not followed by new strategies against A. baumannii.
Because everything proved again ineffective, and given that a single terminal decontamination is not enough, an intervention of thorough cleaning maintained in time at the beginning of April 2016 was proposed. When a patient was discharged, the ICU examination room would be locked for two full days. During that time, in morning and afternoon shifts, a total of four terminal decontaminations would be conducted. If the patient had been colonized/infected by MRM or was a risk patient, six terminal decontaminations would be conducted and registered in a document that would be signed by the nurse/nurse assistant, and personnel responsible for the decontamination. If the microorganism isolated was A. baumannii the same cycles of cleaning were implemented but in a variable way and depending on bed availability - sometimes even up to 12 terminal decontaminations would be conducted. Also hydrogen peroxide disinfection would be used in a very restricted way, since most of the examination rooms did not have doors, which is why the hydrogen peroxide disinfection could only be conducted in 8 out of the 38 examination rooms and in a limited way during 1–2 days/month. During the month of July, the last patients who still remained colonized/infected were kept in isolation in a special unit, and during the months of July, August, and September, when the volume of patients was lower, we proceeded to disinfect using hydrogen peroxide in three out of the four ICU examination rooms.
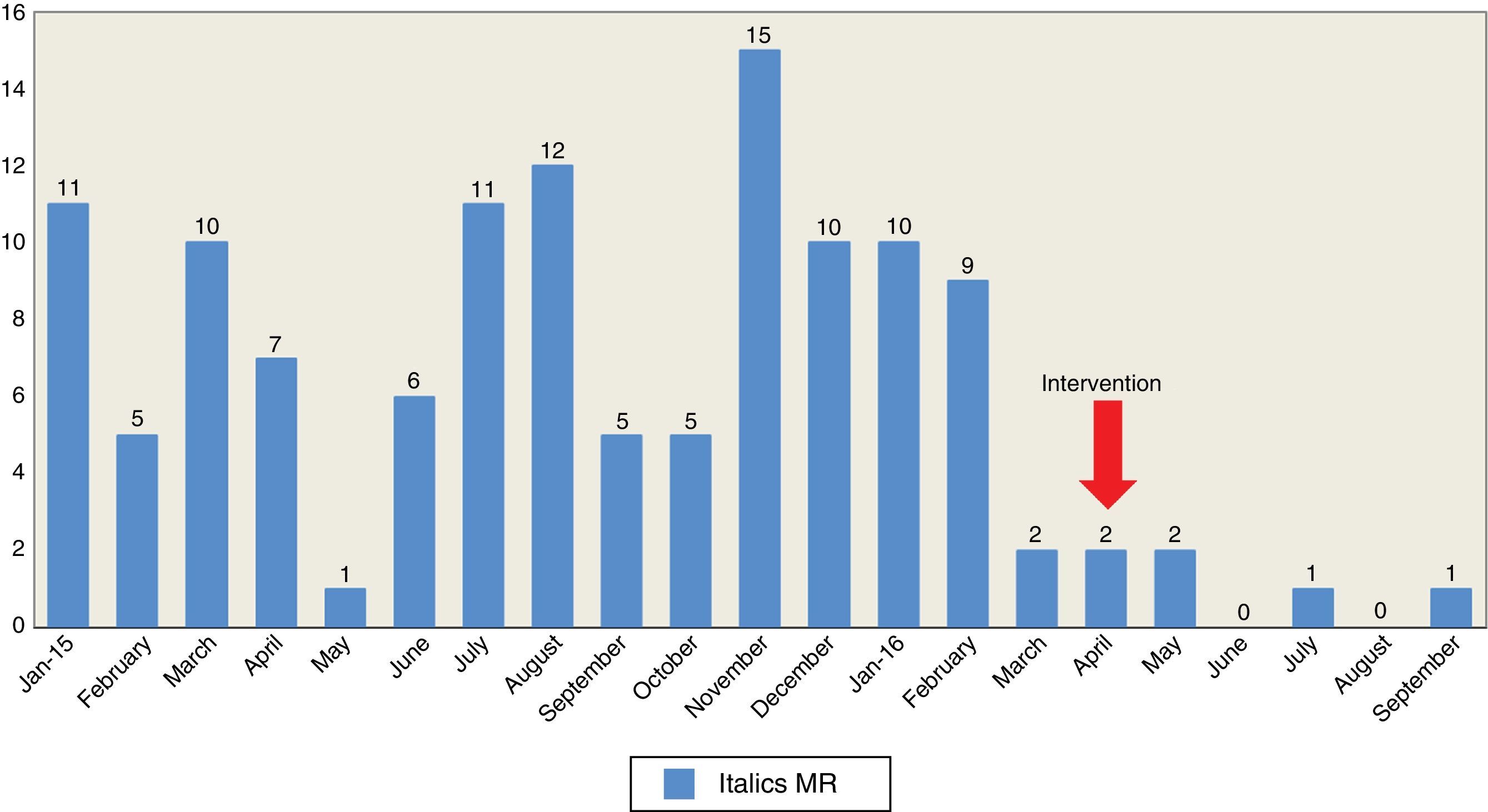
After the implementation of this thorough campaign of decontamination, A. baumannii decreased significantly from 98 patients colonized/infected in 2015 (rate of 9.8 patients/1000 days of hospital stay) to 27 in 2016 – September included (rate 3.9 patients/1000 days of hospital stay), out of which 21 were patients from the 1st trimester of the year, that is, before starting the decontamination campaign. In a six-month period before and after the intervention, from October 2015 through March 2016, the number of colonized/infected patients was 51 (rate 9.7 patients/1000 days of hospital stay) compared to 6 patients for the months of April through September 2016 (rate 1.4 patients/1000 days of hospital stay). One of the goals of the Resistance Zero initiative was to reduce to 20 per cent the rate of patients with MRM infections. In our ICU thanks to the intervention of thorough decontamination and compared to a 6-month period before and after the intervention, we reduced the number of colonized/infected patients by 88.3 per cent. The decrease of A. baumannii lead to significant reductions in the healthcare costs of colistin. In the year 2015, 8380 vials (80mg) were used at €46.146 versus 4274 vials at €23.961 until September 2016, even when the spending from 2016 is attributed to the 1st trimester before the intervention started. Fig. 1 shows the number of colonized/infected patients due to A. baumannii during 2015–2016.
The early detection, isolation of colonized patients, and environmental decontamination are some of the measures recommended in order to control A. baumannii outbreaks.1,7,9,10 In our case, these routine measures proved ineffective for years. Only the additional implementation of a thorough environmental decontamination campaign maintained through time and complemented by specific disinfections using hydrogen peroxide controlled the endemic situation.
Improving the environmental decontamination is one of the interventions recommended as a key intervention to mitigate A. baumannii transmissions. We know it and it is obvious, but we still need to reinforce it and keep working actively if we want to control these outbreaks in the ICU setting. In our own experience, the old message of implementing a truly thorough environmental decontamination campaign—constant through time and in a protocolized way worked satisfactory.
We wish to thank the personnel at the ICU as well as the team of cleaners from the ICU for their effort and determination while trying to control the endemia of Acinetobacter baumannii.
Please cite this article as: Escudero D, Cofiño L, Forcelledo L, Quindós B, Calleja C, Martín L. Control de una endemia de Acinetobacter baumannii multirresistente en la UCI. Recordando lo obvio. Med Intensiva. 2017;41:497–499.