Mechanical ventilation (MV) is a crucial element in the management of acute respiratory distress syndrome (ARDS), because there is high level evidence that a low tidal volume of 6ml/kg (protective ventilation) improves survival. In these patients with refractory respiratory insufficiency, venovenous extracorporeal membrane oxygenation (ECMO) can be used. This salvage technique improves oxygenation, promotes CO2 clearance, and facilitates protective and ultraprotective MV, potentially minimizing ventilation-induced lung injury.
Although numerous trials have investigated different ventilation strategies in patients with ARDS, consensus is lacking on the optimal MV settings during venovenous ECMO. Although the concept of “lung rest” was introduced years ago, there are no evidence-based guidelines on its use in application to MV in patients supported by ECMO. How MV in ECMO patients can promote lung recovery and weaning from ventilation is not clear.
The purpose of this review is to describe the ventilation strategies used during venovenous ECMO in clinical practice.
La ventilación mecánica (VM) es clave en el manejo del síndrome de distrés respiratorio del adulto (SDRA) ya que existe un alto nivel de evidencia de que la ventilación con volúmenes tidales de 6ml/kg (ventilación protectora) mejora la supervivencia. La oxigenación con membrana extracorpórea (ECMO) veno-venosa es una terapia de rescate en el tratamiento de la insuficiencia respiratoria refractaria que mejora la oxigenación, reduce el CO2 y facilita la aplicación de VM protectora, reduciendo potencialmente la lesión pulmonar asociada a VM.
Aunque las estrategias ventilatorias en pacientes con SDRA han sido analizadas en numerosos estudios, no existe consenso respecto a cómo ventilar a pacientes con ECMO veno-venosa. El concepto de «lung rest», introducido hace años, carece aún de evidencias para recomendar su uso pero podría promover la recuperación pulmonar y facilitar el destete de la VM.
El objetivo de esta revisión es describir las diferentes estrategias de ventilación en pacientes tratados con ECMO veno-venosa.
Mechanical ventilation (MV) constitutes the basis of management in patients with acute respiratory distress syndrome (ARDS) and respiratory failure of other origins. To date, only MV with low tidal volume (Vt) and limitation of plateau pressure (Pp) has been found to reduce mortality in these patients.1 In more recent studies, MV in prone decubitus has been observed to improve survival in patients with severe ARDS.2
Despite the technological advances in recent years, mortality due to ARDS remains high (40–50%).3 In the more serious presentations of the syndrome, with PaO2/FiO2≤100mmHg, according to the new definition of Berlin,4 the expectable mortality rate would be 45%, though historically it has been reported that mortality can exceed 60%.5 However, in a recent study involving 98 patients with ARDS, the in-hospital mortality rate was 37.7%, and did not differ between moderate and severe ARDS. In this regard, mortality was seen to depend on other factors such as Pp>30cmH2O during the first 72h.6
Extracorporeal membrane oxygenation (ECMO) allows ventilatory support in the form of venovenous ECMO (VV ECMO) or cardiac and respiratory support through veno-arterial ECMO (VA ECMO). The use of ECMO has increased in recent years, fundamentally due to the good outcomes obtained with the technique (particularly with VV ECMO) in the influenza A (H1N1) epidemic, where survival rates of 77% were reached with ECMO in reference centers,7 and also as a consequence of the technological improvements (use of centrifuge pumps, oxygenation membranes of longer duration), greater biocompatibility of the systems, and lesser anticoagulation needs.8
Extracorporeal membrane oxygenation is a rescue strategy in refractory respiratory failure that affords oxygenation and CO2 clearance. However, it also allows us to apply a protective (Vt 4–8ml/kg ideal b.w., Pp<28–30cmH2O) or “ultraprotective” ventilation strategy (Vt≤4ml/kg ideal b.w., Pp<25cmH2O) in order to minimize ventilator-induced lung injury (VILI).9 Carbon dioxide removal (ECCO2R) is simpler than VV ECMO, and has been shown to lower the concentration of cytokines at pulmonary level (in bronchoalveolar lavage [BAL]) when ventilating with Vt≤6ml/kg ideal b.w., thereby affording effective elimination of CO2.10 These systems effectively clear CO2 in patients with hypercapnia of different etiologies, but also allow ultraprotective ventilation in patients with severe ARDS – the benefits of which remain to be determined.11 In the absence of VV ECMO or ECCO2R, we would be obliged to accept permissive hypercapnia and/or hypoxemia levels that are not clearly established. Nevertheless, although this type of ventilation with VV ECMO or ECCO2R appears promising, no beneficial impact in terms of mortality has yet been established,12 and currently most centers place priority on weaning from extracorporeal devices versus weaning from the ventilator.13
There are no clinical evidence-based guidelines recommending a concrete form of ventilation in patients subjected to VV ECMO, though 77% of the centers with experience apply the “lung rest” concept, with low Vt, low respiratory frequency (RF), and high positive end-expiratory pressure (PEEP).13
Ventilator-induced lung injuryVentilator-induced lung injury occurs at four levels. The use of high plateau pressures induces barotrauma; injury derived from ventilation with high Vt causes volutrauma; and the activation of certain inflammatory processes in the alveolar endothelial and epithelial cells induced by aggressive MV gives rise to biotrauma. Furthermore, the cyclic opening and closing of the alveolar units produces atelectrauma—the latter being defined as the percentage of collapsed lung that opens at the end of inspiration and collapses again at the end of expiration.14
The lung with ARDS is heterogeneous. Studies involving thoracic computed tomography (CT) have demonstrated the existence of areas with collapsed alveoli in dependent zones and others with ventilated alveoli in non-dependent zones, which are those that receive most of the Vt. The use of protective ventilation with Vt 6ml/kg produces alveolar overdistension in almost 30% of all cases of ARDS.1 Patients with ARDS and treated with ECMO are intensely hypoxemic, with large zones of collapsed lung, often affecting all four lung quadrants. As a result, the ventilated alveoli receive most of the Vt and are subject to overdistension despite the adoption of protective ventilation measures.15 However, it does not seem that Vt exclusively plays an important role in the development of VILI, though we need regional and dynamic data on pulmonary ventilation and circulation in patients with ARDS in order to evaluate the application of different ventilation strategies. In this regard, and in addition to thoracic CT, electrical impedance tomography (EIT) affords real-time visioning of regional ventilation and produces information about how the different ventilatory parameters affect the lung, with a view to minimizing VILI.16
The degree to which cyclic alveolar opening and closing, and alveolar overdistension, contribute to VILI is not clear. A study involving patients with acute lung injury (ALI) and ARDS treated with high PEEP measured alveolar inflammatory activity using positron-emission tomography (PET) and alveolar distension with CT at the end of inspiration and at the end of expiration. Inflammation in the ventilated lung regions and in the rest of the lung was related to Pp>26–27cmH2O, though no association was found between metabolic activity and cyclic alveolar opening and closing.17
The use of high PEEP to improve alveolar recruitment, minimizing alveolar overdistension, has not been shown to reduce mortality in patients with ARDS.18 In patients treated with ECMO, the use of high PEEP (>10cmH2O) in the first three days of extracorporeal support was independently correlated to a decrease in mortality.19
Therefore, a protective ventilation strategy should always be adopted in patients with ARDS with or without ECMO, though we do not know how to use PEEP for reducing VILI in these individuals, where the existence of pulmonary heterogeneity is a fact. The increase in PEEP protects the lungs, provided it is accompanied by a change in lung mechanics, i.e., the same Vt should result in improved respiratory compliance (CRS).20 In this regard, studies involving high PEEP have not demonstrated benefits in terms of survival,21 though PEEP has been shown to offer benefit in patients with increased lung recruitability.22
A decrease in driving pressure (ΔP=Vt/CRS) is the factor that has been associated to improved survival in ARDS patients, considering that CRS is closely related to the aerated lung volume, i.e., functional lung size.20 Calculated as Pp-PEEP (cmH2O), a decrease in ΔP is associated to an improved prognosis, while an increase is associated to a poorer prognosis.
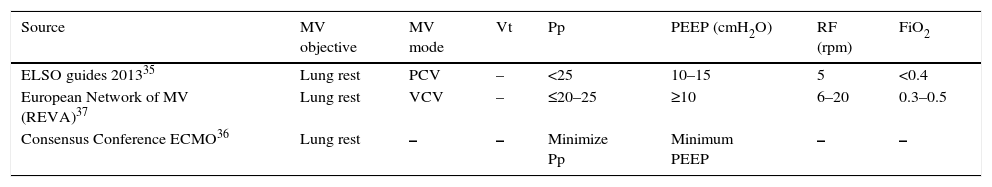
Mode of mechanical ventilation with extracorporeal membrane oxygenationThe MV mode to be used in patients subjected to ECMO has not been established, and no comparative studies are available. Table 1 shows the different ventilation modes in patients subjected to VV ECMO, according to the opinion of different expert sources.
Mechanical ventilation in VV ECMO according to the opinion of different expert sources.
| Source | MV objective | MV mode | Vt | Pp | PEEP (cmH2O) | RF (rpm) | FiO2 |
|---|---|---|---|---|---|---|---|
| ELSO guides 201335 | Lung rest | PCV | – | <25 | 10–15 | 5 | <0.4 |
| European Network of MV (REVA)37 | Lung rest | VCV | – | ≤20–25 | ≥10 | 6–20 | 0.3–0.5 |
| Consensus Conference ECMO36 | Lung rest | – | – | Minimize Pp | Minimum PEEP | – | – |
PEEP: positive end-expiratory pressure; Pp: plateau pressure; FiO2: inspired fraction of oxygen; RF: respiratory frequency; REVA: Réseau Europeen de Recherche en Ventilation Artificielle; PCV: pressure control ventilation; VCV: volume control ventilation; MV: mechanical ventilation; Vt: tidal volume.
In a recent international study involving 141 centers from 28 countries on all continents and belonging to the Extracorporeal Life Support Organization (ELSO), most of the centers (62%) were seen to use controlled MV modes, while only 27% used assist modes in patients with VV ECMO.13 In the international study recently published by Camporota et al., involving 133 surveyed centers, the pressure control mode was used in 64.4% of the centers, the pressure assist mode in 47.3%, bilevel positive airway pressure (BiPAP) in 17.1%, volume control mode in 11.6%, airway pressure release ventilation (APRV) in 4.6%, and neurally adjusted ventilatory assist (NAVA) and high-frequency ventilation in 1% of the centers.23 Lastly, in a multicenter study in three hospitals in Australia and France, 55% of the patients subjected to ECMO (VV ECMO the 98% of the cases) received pressure control modes.19
In general, pressure modes allow daily monitoring of Vt, providing information on the improvement or worsening of lung compliance, and make it possible to increase Vt to at least 6ml/kg for starting weaning from VV ECMO. The inconvenience is that the limitation of Vt may prove difficult in assist mode.24 The APRV mode requires spontaneous ventilation of the patient, and may be an alternative to the pressure control mode in patients with spontaneous ventilation.15
In the early stages of ARDS it is more difficult to maintain spontaneous ventilation, and the patient is often under neuromuscular block. After this phase, spontaneous ventilation allows respiratory muscle and diaphragmatic training, with a decrease in the sedation requirements, though when poorly applied it can worsen patient-ventilator asynchrony.24 The NAVA mode in patients subjected to ECMO reduces patient-ventilator asynchrony in the lung function recovery phase.25
Tidal volume and plateau pressure with extracorporeal membrane oxygenationOf the centers belonging to the ELSO, and with regard to the Vt used, 76% claimed to ventilate their patients with Vt≤6ml/kg, while 21% specified no Vt value during support with VV ECMO.13 In animals, a reduction of Vt<4ml/kg ideal b.w. has been associated to a decrease in lung edema and injury.26 In the Xtravent multicenter study, “ultraprotective ventilation” with ECCO2R versus protective ventilation did not result in a decrease in the days of MV, though a reduction of alveolar interleuking-6 levels was observed,12 as previously reported.10 In the post hoc analysis, in the group of patients with PaO2/FiO2≤150mmHg, a decrease was recorded in the days of MV in the group subjected to ECCO2R (40.9±12.8 vs 28.2±16.4; p=0.033).12
According to a recent systematic review involving 2042 patients in 49 studies, the tendency is to use an “ultraprotective” strategy, ventilating with Vt≤4ml/kg in order to limit Pp to ≤30cmH2O. This practice possibly reflects the impossibility of maintaining adequate ventilation without a risk of lung injury before starting extracorporeal support.26 In another international survey, 31% of the centers were seen to ventilate patients with ECMO using an “ultraprotective” strategy.13 According to different studies, the way in which we use MV during support with VV ECMO appears to have an impact upon mortality.15,26,27 The study published by Pham et al. shows the reduction of Pp on the first day of ECMO to be independently associated to lowered mortality.28 Likewise, in an international multicenter study, one of the predictors of mortality in patients treated with ECMO was Pp>30cmH2O before introducing ECMO.19
The Predicting death for severe ARDS on VV-ECMO (PRESERVE) mortality risk score takes 8 pre-ECMO parameters into account in predicting the probability of survival in patients with severe ARDS treated with VV ECMO. Patient age, body mass index, immunosuppression, the use of prone decubitus, the days of MV, the SOFA score, PEEP and Pp>30cmH2O were the calculated parameters.29
There are no recommendations on how to proceed in lowering Vt once VV ECMO support has been started. A reduction in the first 1–3 days of support to <4ml/kg ideal b.w. could be proposed.19 In a recent study, only the parameter Pp≤31cmH2O was associated to in-hospital survival.30
Positive end-expiratory pressure with extracorporeal membrane oxygenationIn patients with ARDS, PEEP is used to maintain alveolar recruitment, improve oxygenation and prevent VALI (atelectrauma with cyclic alveolar opening and closing). However, alveolar overdistension and the increase in right ventricular afterload are deleterious effects of the indiscriminate use of PEEP.
The PEEP level to be used in patients treated with ECMO is subject to controversy. The reduction of Vt, particularly when “ultraprotective ventilation” is used (Vt<4ml/kg ideal b.w.), can produce atelectasis with worsening of the ventilation/perfusion ratio. The ELSO therefore recommends a PEEP level of 10cmH2O, in contrast to the protocol established by Richard et al. in the Consensus Conference, where the recommendation is simply to use “minimum PEEP” for “minimum Pp” (Table 1). Higher PEEP levels could cause alveolar overdistension,18 as well as reduce venous return in patients with VV ECMO and worsen right ventricular function in VA ECMO.15
In patients with VV ECMO, PEEP is not necessary to improve oxygenation, except in intensely hypoxemic individuals or cases requiring high blood flows (>5l/min) during support (e.g., septic shock patients). In contrast to VV ECMO, the ECCO2R systems require PEEP and FiO2 in the respirator in order to improve oxygenation, since they operate with a much lower blood flow than VV ECMO, and CO2 clearance is fundamentally dependent upon the flow of gas.11,31,32
In ECMO, the use of high PEEP levels would favor alveolar recruitment, which in turn could accelerate lung recovery through the prevention of capillary loss and macrophage activation generated in lung hypoxemic regions induced by the presence of atelectasis.10,28,33
In an international survey involving 133 centers, 63% used a fixed PEEP level, 21% introduced adjustments according to compliance, 9.3% did so according to the radiological findings, and 7.3% introduced adjustments according to EIT. In turn, 34.9% of the centers used PEEP≥10cmH2O, while 27.9% used PEEP levels below this value. Only 15.5% used PEEP 15–20cmH2O.33
As has been commented above, in a retrospective study, the use of PEEP<10cmH2O during the first three days of treatment with ECMO was associated to increased mortality. On the other hand, in a more recent retrospective study involving 62 patients, a one-point PEEP increment was seen to be associated to a 36.2% decrease in the odds ratio for survival 30 days after discharge (95%CI 10.8–54.4%; p=0.009)(PEEP survivors 8.5±2, survivors 7.3±2; p=0.04).30 In patients exposed to increased overdistension, the effect of PEEP may prove deleterious, and in patients with more recruitable areas we could perhaps use higher PEEP levels.34
It therefore would be very difficult to recommend a PEEP level in these patients. Table 1 shows the different recommendations of groups of experts. The latest ELSO guides warn that “in patients with respiratory failure and ECMO, it could be a mistake to try to recruit lung volume in the early stages”. This would speak in favor of the risk of pulmonary overdistension in certain patients, recommending PEEP levels of between 5 and 15cmH2O.35 In the same sense, according to the only European Consensus Conference on ECMO of 2014, the recommendation for ventilating ARDS patients with ECMO would be to “adjust MV to minimize Pp while we apply minimum PEEP”.36 In patients with ARDS due to influenza A (H1N1), the Réseau Europeen de Recherche en Ventilation Artificielle (REVA) recommended lowering Vt to maintain Pp≤20–25cmH2O, with the application of PEEP≥10cmH2O.37
As has been commented above, driving pressure (ΔP=Pp−PEEP) is the factor that has been associated to improved survival in ARDS patients.20 In patients with ECMO, previous studies have shown high ΔP to be associated to poorer survival.11,19 In a recent study carried out to assess the association among the different ventilatory parameters during ECMO indicated for refractory hypoxemia in ARDS, the authors concluded that increased ΔP is the only ventilatory parameter during ECMO showing an independent association to in-hospital mortality in these patients.38 In this study, Serpa-Neto et al. included 545 patients from 9 studies, where in addition to ΔP, advanced age, the male sex and a low body mass index were also seen to be independently associated to mortality.
Respiratory frequencyThe RF to be used in patients subjected to ECMO has likewise not been established. The ELSO recommended a low RF (4–5rpm) to avoid mechanical lung “stress”, though in general the range is between 4 and 30rpm as evidenced by the literature.15 In general, the adjustment of RF aims to ensure maintenance of the arterial pH, but this concept changes in patients subjected to VV ECMO, where maintenance of the arterial pH and PaCO2 are directly dependent upon the flow of gas in the oxygenation membrane. In this context, PaCO2 is to be slowly reduced once support has been started, with a gas/blood flow of 1:1 in ECMO.31,32
In a recent international survey, 55% of the centers ventilated their patients subjected to ECMO with a RF of 5–10rpm,23 possibly reflecting a tendency to ventilate with lower RF as in the “lung rest” model proposed by Gattinoni et al. years ago, and in which ventilation with a low RF (3–5rpm) and a low peak inspiratory pressure with an ECCO2R system in patients suffering severe ARDS of pulmonary origin resulted in improved lung function in 72.8% of the patients.34 In this regard, the latest recommendations of the ELSO are to “use MV with low parameters in order to allow lung rest”.35
Fraction of inspired O2 in patients with extracorporeal membrane oxygenationIt is clear that in order to minimize VILI we must lower FiO2 to minimum levels, with the purpose of maintaining SatpO2>80% in VV ECMO,31 or between 84 and 88% according to the different groups.35,37
ConclusionsIn patients with ARDS, MV with the reduction of Pp has been shown to reduce mortality. However, there are no clear evidences, guides or recommendations on how to ventilate patients subjected to ECMO. A decrease in driving pressure has been associated to improved in-hospital survival among patients with severe ARDS treated with ECMO. The monitoring of this MV parameter therefore could be recommended in the same way as in patients without ECMO.
Since extracorporeal systems oxygenate and clear CO2, protective and “ultraprotective” MV is possible, and would allow a reduction of VILI in these patients. The lowering of Vt (<4ml/kg ideal b.w.), Pp (<25cmH2O), PEEP (5–15cmH2O) and RF allows us to maintain lung rest, avoiding alveolar overdistension, biotrauma and atelectrauma. Further studies are needed to demonstrate the impact of this ventilatory strategy upon the mortality of patients with severe ARDS treated with VV ECMO or ECCO2R systems.
Conflicts of interestThere are no conflicts of interest.
None.
Please cite this article as: López Sanchez M. Ventilación mecánica en pacientes tratados con membrana de oxigenación extracorpórea (ECMO). Med Intensiva. 2017;41:491–496.