We evaluated the effect of changes in FiO2 on the bias and accuracy of the determination of oxygen consumption (V⋅O2) and carbon dioxide production (V⋅CO2) using the E-COVX monitor in patients with mechanical ventilation.
DesignDescriptive of concordance.
SettingIntensive Care Unit.
Patients or participantsPatients with mechanical ventilation.
InterventionsWe measured V⋅O2 and V⋅CO2 using the E-COVX monitor. Values recorded were the average in 5min. Two groups of 30 patients. We analyzed: 1) the reproducibility in the measurement of V⋅O2 and V⋅CO2 at FiO2 0.4, and 2) the effect of the changes in FiO2 on the measurement of V⋅O2 and V⋅CO2. Statistical analysis was performed using Bland and Altman test.
Variables of main interestBias and accuracy.
Results1) FiO2 0.4 reproducibility: The bias in the measurement of V⋅O2 and V⋅CO2 was 1.6 and 2.1mL/min, respectively, and accuracy was 9.7 to −8.3% and 7.2 to −5.2%, respectively, and 2) effect of FiO2 on V⋅O2: The bias of V⋅O2 measured at FiO2 0.4 and 0.6 was −4.0mL/min and FiO2 0.4 and 0.8 was 5.2mL/min. Accuracy between FiO2 0.4 and 0.6 was 11.9 to −14.1%, and between FiO2 0.4 and 0.8 was 43.9 to −39.7%.
ConclusionsThe E-COVX monitor evaluates V⋅O2 and V⋅CO2 in critical patients with mechanical ventilation with a clinically acceptable accuracy until FiO2 0.6.
Valorar el efecto de la FiO2 sobre el sesgo y la precisión en la medición del consumo de oxígeno (V⋅O2) y la producción de dióxido de carbono (V⋅CO2) con el monitor E-COVX en pacientes con ventilación mecánica.
DiseñoDescriptivo de concordancia.
ÁmbitoUnidad de Cuidados Intensivos.
Pacientes o participantesPacientes con ventilación mecánica.
IntervencionesSe midieron el V⋅O2 y la V⋅CO2 con el monitor E-COVX. Los valores de V⋅O2 y V⋅CO2 fueron el promedio de 5min. Dos grupos de 30 pacientes. Se analizó: 1) la reproducibilidad de la medición del V⋅O2 y la V⋅CO2 con una FiO2 de 0,4, y 2) el efecto de los cambios en la FiO2 sobre el V⋅O2 y la V⋅CO2. Análisis estadístico por el método de Bland y Altman.
Variables de interés principalesSesgo y precisión.
Resultados1) Reproducibilidad a una FiO2 de 0,4: los sesgos en la medición del V⋅O2 y la V⋅CO2 fueron de 1,6 y 2,1mL/min, respectivamente, y los errores en la precisión fueron de 9,7 a −8,3% y de 7,2 a −5,2%, respectivamente, y 2) efecto de la FiO2 sobre el V⋅O2: el sesgo del V⋅O2 medido a una FiO2 de 0,4 y 0,6 fue de −4,0mL/min y a FiO2 de 0,4 y 0,8, de 5,2mL/min. La precisión entre FiO2 de 0,4 y 0,6 fue de 11,9 a −14,1%, y entre FiO2 de 0,4 y 0,8, de 43,9 a −39,7%.
ConclusionesEl monitor E-COVX mide el V⋅O2 y la V⋅CO2 en pacientes críticos con ventilación mecánica con un sesgo y una precisión clínicamente aceptables hasta una FiO2 de 0,6.
The main interest of measuring oxygen consumption (VO2) and the production of carbon dioxide (VCO2) in critical patients subjected to mechanical ventilation (MV) is to calculate energy expenditure by applying the formula of Weir.1 Recent studies have shown that a calorie supply capable of compensating the losses resulting from energy expenditure shortens the duration of mechanical ventilation, reduces the nosocomial infection rate, facilitates physical recovery and reduces mortality.2–5 The measurement of VO2 and VCO2 also has other applications, however. In effect, the measurement of VO2 allows us to assess the relationship between oxygen transport and VO26 or determine the respiratory effort of a given ventilatory mode with respect to some other mode.7 The measurement of VCO2 in turn allows us to measure the physiological dead space.8
However, the precise measurement of VO2 and VCO2 in the critical patient subjected to mechanical ventilation poses a series of problems including the need for a fraction of inspired oxygen (FiO2) above that of room air, particularly in the acute phase of the disease; airway gas leakage due to the positive pressure of the ventilator; and the presence of water vapor in the expired gas.1,9–11 Of these problems, FiO2 is the most important, since error in the measurement of the concentrations of inspired and expired oxygen in order to determine VO2 is amplified when FiO2 is incremented.12
The measurement of respiratory gas exchange in patients under mechanical ventilation has been facilitated by the development of automated systems capable of measuring VO2 and VCO2 on a breath-to-breath basis. In this regard, some studies have reported that the M-COVX and E-COVX monitors can be used in patients subjected to mechanical ventilation and with a need for high FiO2 (<0.85), with an error acceptable to clinical practice.13–15
The present study was carried out to evaluate the effect of FiO2 upon precision in the measurement of VO2 and VCO2 using the E-COVX metabolic monitor in critical patients subjected to mechanical ventilation.
Material and methodsPatientsThe study included patients admitted to the Intensive Care Unit (ICU), intubated and subjected to mechanical ventilation, who were receiving sedatives (midazolam or propofol) and/or analgesics (morphine or fentanyl) in continuous perfusion. Measurements were made of VO2 and VCO2, with the calculation of resting energy expenditure (REE). The study was carried out in the morning, with the patient under resting conditions, the headrest raised 30 degrees, and after two or more days of mechanical ventilation. All the patients were ventilated in volume control mode with FiO2≤0.4. Before indirect calorimetry measurement, we checked the pressure of the balloon of the endotracheal tube and the absence of air leakage. Indirect calorimetry measurement was carried out during the administration of enteral, parenteral or mixed nutrition, with a calorie supply of 15–30kcal/kg/day. The nutrition was administered continuously and was not interrupted, since the increase in VO2 and VCO2 is constant and with a value of about 3%.16 During at least 30min before the measurements we performed no tracheal aspirations, physiotherapy, postural changes, body hygiene measures, radiological studies or catheter insertions.17,18
The following conditions were regarded as study exclusion criteria: hemodynamic instability (defined as the need to modify vasoactive drug doses or variations >20% in arterial pressure and/or heart rate); a respiratory frequency of over 35rpm; the need for FiO2>0.4; a body temperature of under 36°C or over 38°C; a sedation level as determined with the Richmond Agitation-Sedation Scale19 of over −3; patients with bronchopleural fistulas; and patients subjected to renal replacement therapy.
The study was approved by the hospital research committee. Since the study involved a monitoring technique, the need for informed consent was not considered necessary.
E-COVX metabolic monitorThe E-COVX metabolic monitor (GE Healthcare/Datex-Ohmeda, Helsinki, Finland) is a noninvasive system equipped with a paramagnetic analyzer for oxygen, an infrared analyzer for CO2, and a pneumotachograph for measuring inspired and expired volumes. The pneumotachograph and gas sampling ports were located in a disposable connector called D-Lite sensor (GE Healthcare Finland Oy, Helsinki, Finland), placed between the heat and humidity exchanger (Edith Flex®, GE Healthcare Finland Oy, Helsinki, Finland) and the Y-piece of the ventilator circuit, in order to avoid water accumulation.14 A connector with a dead space of 15ml (the manufacturer recommended a dead space of 5ml) was placed between the D-Lite sensor and the Y-piece. The purpose of this dead space was to avoid contamination of the expired gas with the continuous air flow of the ventilator, which was set to minimum (2l/min).
In order to reduce systematic error in the volume measurements, the E-COVX monitor uses the Haldane transformation to calculate both VO2 and VCO2. Systematic error occurs in all the measurements and is inherent to the apparatus itself or to the measurement process. In contrast, random error is accidental, not controllable and can be reduced by increasing the sample size. The Haldane transformation consists of measuring the inspiratory volume and estimating the expiratory volume, since the latter is dependent upon the temperature (assumed to be 35°C) and humidity (assumed to be 100%) of the expired gas.
The signals from the pneumotachograph and gas analyzers were synchronized in order to allow breath-to-breath gas exchange estimates. The results corresponding to VO2 and VCO2 were expressed each minute as an average of the last 60s. The measurements of VO2 and VCO2 were recorded only when the patient was metabolically stable (defined as a variation of ≤5% in 10 consecutive values).20,21 The volumes were corrected to standard conditions of temperature, pressure and dryness.
The E-COVX monitor is ready for use 5min after being turned on, and automatic calibration is performed. The system calibrations are made every 6 months according to the instructions of the manufacturer, who reports a precision of ±10% for FiO2 <0.7 and a respiratory frequency of <35rpm.
Study protocolTwo groups of 30 patients each were studied sequentially and on a non-consecutive basis: in the first group, we assessed the reproducibility of the measurements of VO2 and VCO2 at FiO2=0.4, while in the second group we evaluated the effect of the changes in FiO2 upon the measurements of VO2 and VCO2. Each VO2 and VCO2 value in the study corresponded to the average of 5min.20,22
In the first group, 30min after turning on the E-COVX monitor and with the ventilator set to FiO2=0.4, we recorded body temperature and the VO2 and VCO2 values corresponding to 5min. Data recording was repeated 30min later in order to establish the reproducibility of the VO2 and VCO2 measurements at FiO2=0.4.
In the second group, 30min after turning on the E-COVX monitor and with the ventilator set to FiO2=0.4, we likewise recorded body temperature and the VO2 and VCO2 values corresponding to 5min. The ventilator was then modified to FiO2=0.6, and after 30min we again recorded body temperature and the VO2 and VCO2 values corresponding to 5min. Lastly, the process was repeated at FiO2=0.8.
Statistical analysisThe descriptive data included the number and percentage corresponding to categorical variables, and the mean and standard deviation or median and interquartile range (IQR) in the case of continuous variables. The Kolmogorov–Smirnov test was used to assess normal distribution of the data. We used the Student t-test or the Friedman test in application to continuous variables, and the χ2 test or the Fisher exact test in the case of categorical variables. The Bland and Altman method23 was used to determine bias (mean difference between two measurements) and precision as the limits of agreement (twice the standard deviation of the difference between two measurements). Bias (or accuracy) assesses the similarity between the mean values of repeated measurements. Precision (reproducibility or variability) refers to the difference between repeated measurements and assesses the degree of dispersion. In addition, we evaluated absolute agreement between the repeated measurements of VO2 and VCO2 using the intraclass correlation coefficient (ICC) with the corresponding 95% confidence interval (95%CI). The error between two measurements was expressed as a percentage of the limits of agreement with respect to the mean value of the two measurements. A priori, an error of < 20% was considered acceptable.24 Statistical significance was considered for p<0.05. The data were analyzed using the SPSS, version 19.0 statistical package (SPSS Inc., Chicago, IL, USA).
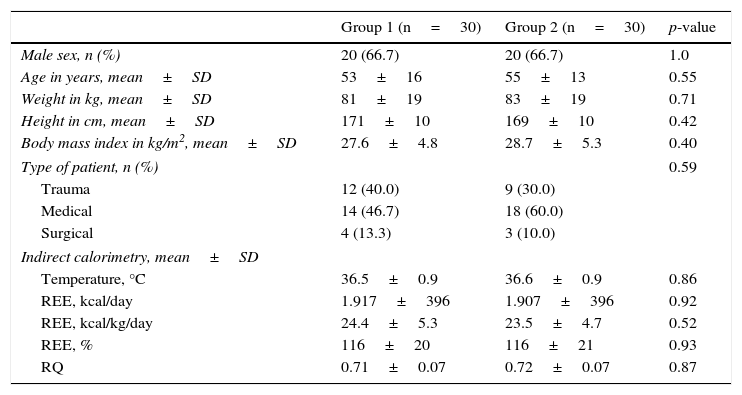
ResultsThere were no demographic, clinical or metabolic activity differences (measured by indirect calorimetry) between the two groups (Table 1).
Demographic and clinical characteristics, and indirect calorimetry results of the two groups of patients.
| Group 1 (n=30) | Group 2 (n=30) | p-value | |
|---|---|---|---|
| Male sex, n (%) | 20 (66.7) | 20 (66.7) | 1.0 |
| Age in years, mean±SD | 53±16 | 55±13 | 0.55 |
| Weight in kg, mean±SD | 81±19 | 83±19 | 0.71 |
| Height in cm, mean±SD | 171±10 | 169±10 | 0.42 |
| Body mass index in kg/m2, mean±SD | 27.6±4.8 | 28.7±5.3 | 0.40 |
| Type of patient, n (%) | 0.59 | ||
| Trauma | 12 (40.0) | 9 (30.0) | |
| Medical | 14 (46.7) | 18 (60.0) | |
| Surgical | 4 (13.3) | 3 (10.0) | |
| Indirect calorimetry, mean±SD | |||
| Temperature, °C | 36.5±0.9 | 36.6±0.9 | 0.86 |
| REE, kcal/day | 1.917±396 | 1.907±396 | 0.92 |
| REE, kcal/kg/day | 24.4±5.3 | 23.5±4.7 | 0.52 |
| REE, % | 116±20 | 116±21 | 0.93 |
| RQ | 0.71±0.07 | 0.72±0.07 | 0.87 |
SD: standard deviation; REE: resting energy expenditure; RQ: respiratory quotient.
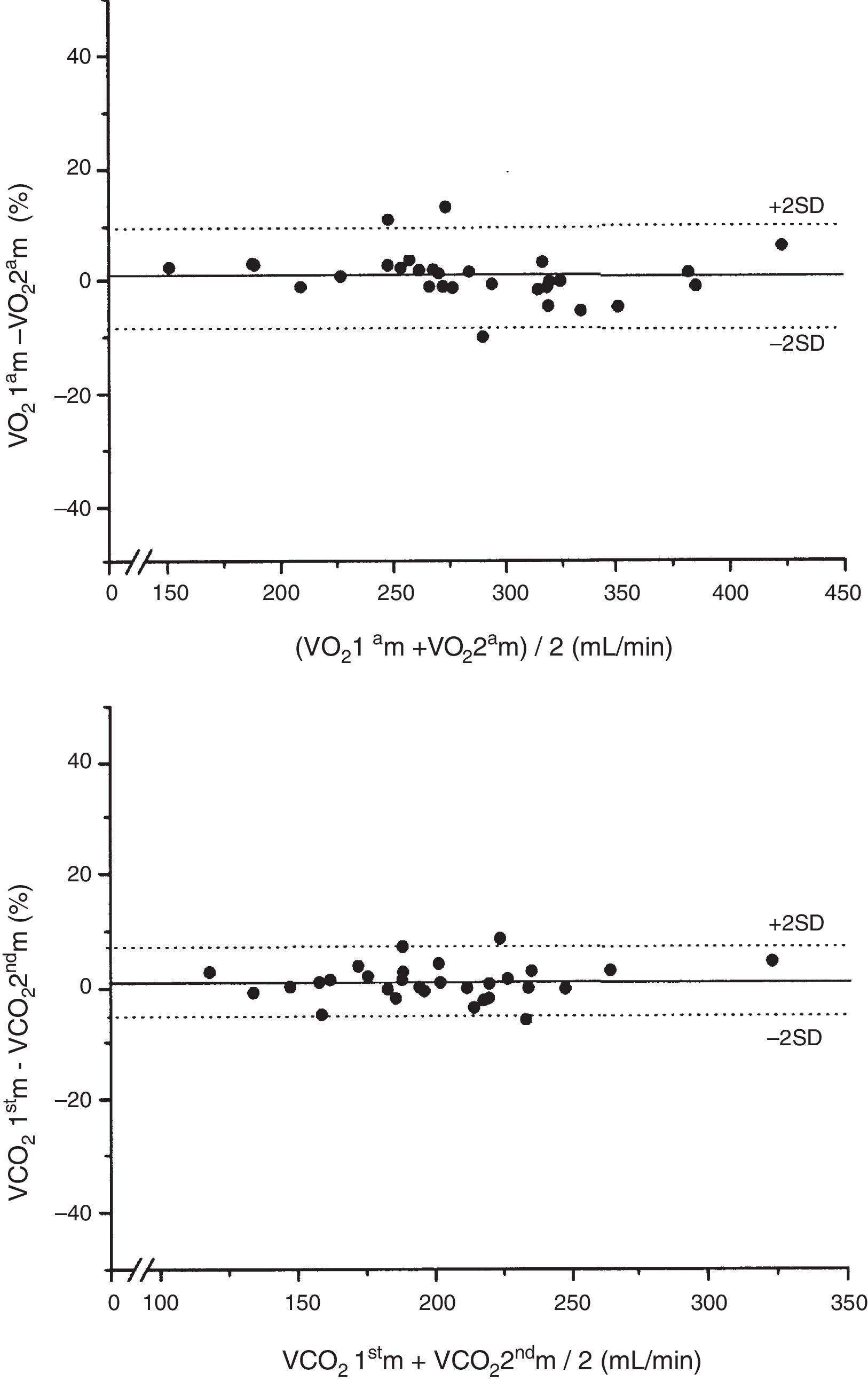
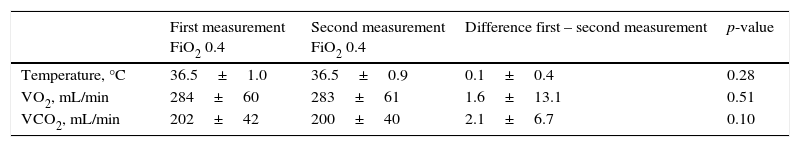
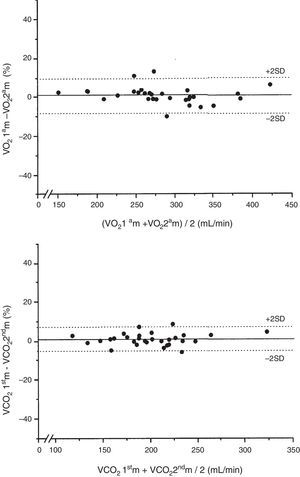
There were no significant differences in body temperature, VO2 or VCO2 between the first and second indirect calorimetry measurements at FiO2=0.4 (Table 2). The biases between the two measurements of VO2 and VCO2 were 1.6 and 2.1mL/min, respectively (Table 2). The precision for VO2 was 27.8 to −24.6mL/min, which represents a percentage error of 9.7 to −8.3%, versus 15.5 to −11.3mL/min for VCO2, which represents a percentage error of 7.2 to −5.2% (Fig. 1). The ICC (95%CI) for VO2 was 0.98 (0.95–0.99), and 0.98 (0.97–0.99) for VCO2.
Reproducibility of the measurements of VO2 and VCO2 at FiO2=0.4.
| First measurement FiO2 0.4 | Second measurement FiO2 0.4 | Difference first – second measurement | p-value | |
|---|---|---|---|---|
| Temperature, °C | 36.5±1.0 | 36.5±0.9 | 0.1±0.4 | 0.28 |
| VO2, mL/min | 284±60 | 283±61 | 1.6±13.1 | 0.51 |
| VCO2, mL/min | 202±42 | 200±40 | 2.1±6.7 | 0.10 |
FiO2: fraction of inspired oxygen; VO2: oxygen consumption; VCO2: production of carbon dioxide.
Data expressed as mean±standard deviation.
There were no significant differences in the values corresponding to body temperature, VO2 or VCO2 measured at FiO2=0.4, 0.6 and 0.8 (Table 3).
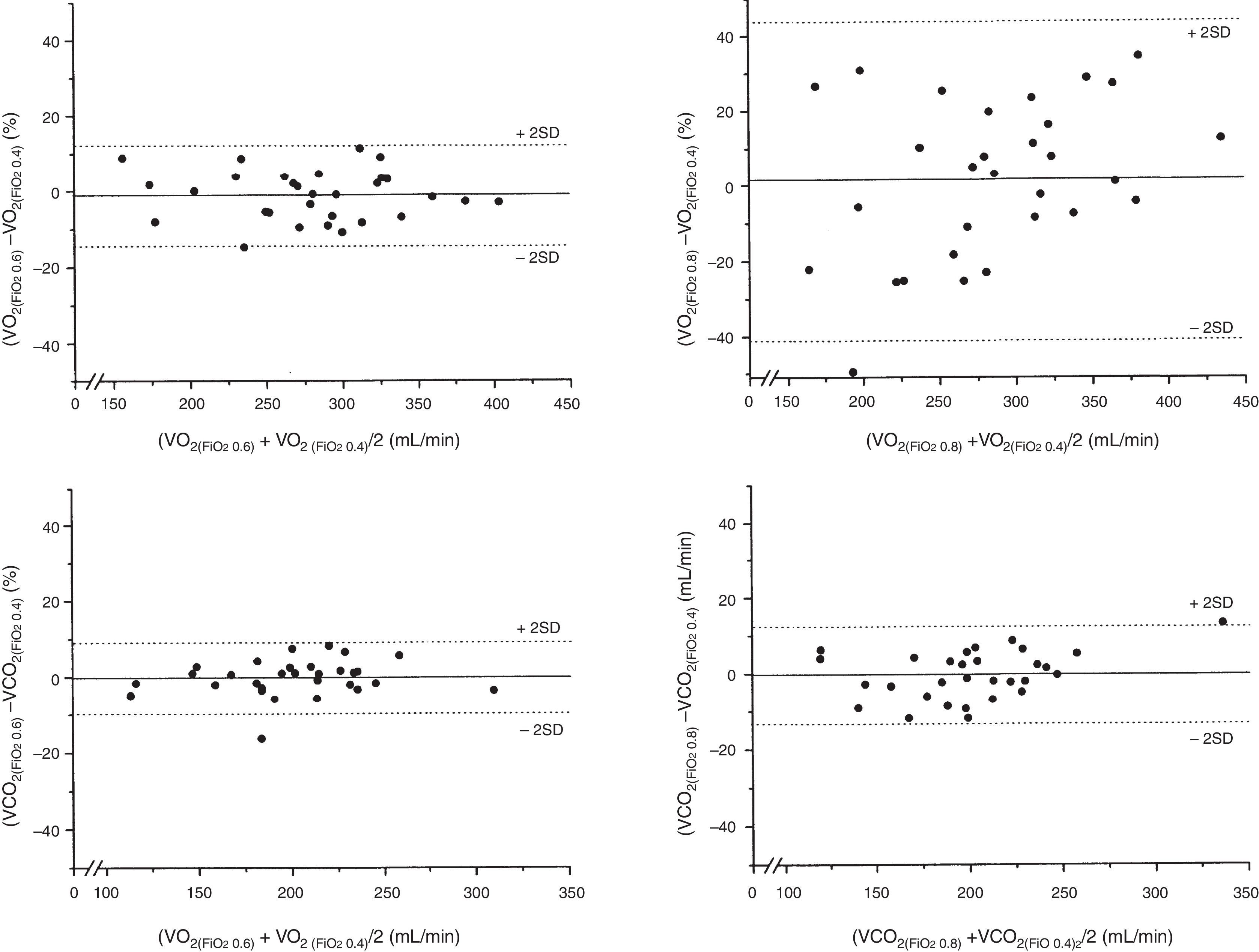
Bias and precision of the measurement of VO2 and VCO2 at FiO2=0.4, 0.6 and 0.8.
| FiO2 0.4 | FiO2 0.6 | FiO2 0.8 | Difference 0.6–0.4 | Difference 0.8–0.4 | p-value | |
|---|---|---|---|---|---|---|
| Temperature, °C | 36.6±0.9 | 36.6±0.9 | 36.6±0.8 | 0.0±0.3 | 0.0±0.4 | 0.99 |
| VO2, mL/min | 283±60 | 279±58 | 288±83 | −4.0±18.1 | 5.2±56 | 0.90 |
| VCO2, mL/min | 201±41 | 201±42 | 201±47 | −0.5±9.8 | −0.2±13.9 | 0.88 |
FiO2: fraction of inspired oxygen; VO2: oxygen consumption; VCO2: production of carbon dioxide.
Data expressed as mean±standard deviation.
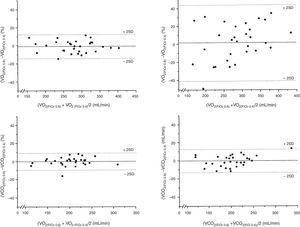
The bias of the VO2 values measured at FiO2=0.4 and 0.6 was −4.0mL/min, while at FiO2=0.4 and 0.8 the bias was 5.2mL/min (Table 3). The precision of the measurements of VO2 between FiO2=0.4 and 0.6 was 32.2 to −40.2mL/min, which represents a percentage error of 11.9 to −14.1%. In turn, the precision of the measurements of VO2 between FiO2=0.4 and 0.8 was 117.2 to −106.8mL/min, which represents a percentage error of 43.9 to −39.7% (Fig. 2). The ICC (95%CI) for VO2 measured at FiO2=0.4 and 0.6 was 0.95 (0.90–0.98), versus 0.70 (0.46–0.85) for VO2 measured at FiO2=0.4 and 0.8.
The bias of the values of VCO2 measured at FiO2=0.4 and 0.6 was −0.5mL/min, while at FiO2=0.4 and 0.8 the bias was −0.2mL/min (Table 3). The precision of the measurements of VCO2 between FiO2=0.4 and 0.6 was 19.5 to −20.5mL/min, which represents a percentage error of 9.3 to −9.9%. In turn, the precision of the measurements of VCO2 between FiO2=0.4 and 0.8 was 27.6 to −28.0mL/min, which represents a percentage error of 12.4 to −13.2% (Fig. 2). The ICC (95%CI) for VCO2 measured at FiO2=0.4 and 0.6 was 0.97 (0.94–0.99), versus 0.95 (0.90–0.98) for VCO2 measured at FiO2=0.4 and 0.8.
DiscussionThe results of our study with the E-COVX metabolic monitor reveal good precision at FiO2=0.4 in the measurement of VO2 and VCO2. We observed no clinically significant bias in the measurements of VO2 and VCO2 over the FiO2 range of 0.4–0.8. However, precision in the measurement of VO2 increased on elevating FiO2 – the situation being clinically inadequate (>20%) with FiO2>0.6. Therefore, in clinical practice we should not use the E-COVX monitor to measure VO2 in critical patients subjected to mechanical ventilation at FiO2>0.6.
The precision of the repeated measurements of VO2 at FiO2=0.4 was 10%, which is consistent with the specifications of the manufacturer, while the precision of VO2 at FiO2=0.6 was about 15%, versus 40% at FiO2=0.8. This progressive and exponential error in precision must be attributed to the increase in FiO2.25 Such a lack of agreement with VO2 measured at FiO2=0.8 is reflected by the low ICC value of only 0.7, while ICC for the measurements of VCO2 always remained above 0.95, independently of the FiO2 setting.
The measurement of VO2 and VCO2 in short periods of time can replace prolonged measurements, with the added advantage of reducing the physiological fluctuations.20,22 This advantage is lost as a result of the sequential design of the study; consequently, precision includes both the physiological variations of metabolism and the true error of the measurements.13 However, the gradual increase in precision of the measurements of VO2 with incrementing FiO2 values, which is not seen with the measurements of VCO2, supports the idea that the increase in the precision of VO2 is due to errors in the measurement of the inspired and expired oxygen concentrations.
Our results contrast with those of other studies that found the measurement of VO2 with the M-COVX monitor at FiO2 settings of up to 0.7 and 0.8 to be clinically acceptable.13–15 These studies are based on the notion that the E-COVX monitor measures VO2 and VCO2 on a breath-to-breath basis for 5min, which would be the equivalent to about 100 measurements (5min at 20rpm). According to the theoretical study of Ultman and Bursztein,12 random error in the measurement of VO2 would be gradually reduced by incrementing the number of measurements. Accordingly, precision is considered to be ±10% when FiO2<0.65, versus ±15% when FiO2 >0.65 and <0.85.25
The results of our study referred to the precision of the measurement of VO2 are consistent with the idea that any error in the measurement of oxygen concentration in the inspired and expired gas is amplified when FiO2 is increased.9,11 An error of 1% in the measurement of FiO2, at FiO2=0.4, results in an error of 15% in the measurement of VO2. At FiO2=0.8 or higher, the same error of 1% results in an error of ≥100%, and because of this we did not perform measurements with FiO2>0.8. On the other hand, the measurement of REE in patients subjected to mechanical ventilation at FiO2>0.6 remains difficult and should not be made. As expected, the precision in the measurement of VCO2 showed minimum changes with increments of FiO2.12
The mean respiratory quotient (RQ=0.72) observed in our series of patients was lower than expected. The RQ in patients subjected to mechanical ventilation under the effects of sedoanalgesia and with enteral, parenteral or mixed nutrition including carbohydrates (50%), lipids (30%) and proteins (20%), should be between 0.8 and 0.9. The most likely explanation for the low RQ would be systematic error in measuring VCO2. In this sense, Meyer et al.26 recorded a VCO2 value with the M-COVX monitor of under 17.6% with respect to the Deltatrac II system. The low RQ could also be due to overestimation of VO2, but this would give rise to a high REE value which we did not observe, since in the formula of Weir for calculating REE, the VO2 multiplying factor is 3.9, versus 1.1 in the case of VCO2.1 The mean REE of our 60 patients was similar to that recorded in other studies in patients with similar demographic characteristics using other measurement methods.5,27
The underestimation of VCO2 has little impact upon measurement of the REE, but precludes the correct interpretation of RQ in assessing the metabolic substrates. Furthermore, it disables calculation of the physiological dead space. A possible source of systematic error is the continuous flow of the ventilator (Engström Carestation), which could dilute the expired gas. However, and despite increasing the dead space between the D-Lite and the ventilator to 15ml (the recommended value being 5ml), we observed no increase in RQ.
The main limitation of our study, apart from its sequential design, is the fact that the measurements of VO2 and VCO2 were not compared with another indirect calorimetry method, such as the Douglas bag, particularly for checking the values of VCO2.
In conclusion, the E-COVX metabolic monitor measures VO2 in critical patients subjected to mechanical ventilation with clinically acceptable precision to a FiO2 setting of 0.6. The measurement of VCO2 is not affected by FiO2.
AuthorshipMireia Ferreruela: data collection, preparation and review of the manuscript.
Joan Maria Raurich: literature search, data collection, study design, data analysis, preparation and final review of the manuscript.
Juan Antonio Llompart-Pou: preparation and final review of the manuscript.
Asunción Colomar: data collection, preparation and review of the manuscript.
Ignacio Ayestarán: data collection, preparation and review of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest in this study.
Please cite this article as: Ferreruela M, Raurich JM, Llompart-Pou JA, Colomar A, Ayestarán I. Efecto de la FiO2 sobre la medición del VO2 y la VCO2 con el monitor metabólico E-COVX. Med Intensiva. 2017;41:461–467.