To comprehensively assess peer-reviewed studies using volatile (VA) or intravenous (i/v) anesthetics for sedation in intensive care units (ICUs), with the hypothesis that the type of sedation may have an impact on survival and other clinically relevant outcomes.
DesignSystematic review and meta-analysis of randomized and non-randomized trials.
SettingICUs.
ParticipantsCritically ill and postoperative patients.
InterventionsNone.
Measurements and main resultsStudies comparing VA versus i/v anesthetics used in the ICU settings were independently systematically searched. Finally, 15 studies (1520 patients of predominantly surgical profile needed VA sedation for less than 96h) were included. VA had no impact on all-cause mortality (very low quality of evidence, Odds Ratio=0.82 [0.60–1.12], p=0.20). However, VA were associated with a reduction in duration of mechanical ventilation (p=0.03) and increase in ventilator-free days (p<0.001). VA also reduced postoperative levels of cardiac troponin (24h), time to extubation (p<0.001) and awakening (p=0.04).
ConclusionsIn this meta-analysis, volatile sedation vs propofol caused the increase in ventilator-free days, the reduction in the duration of mechanical ventilation, time to extubation and the troponin release in medical or surgical ICU patients, while in surgical ICU patients the time to awakening was shortened.
Evaluar exhaustivamente los estudios revisados por pares que utilizan anestésicos volátiles (AV) o intravenosos (iv) para sedación en unidades de cuidados intensivos (UCI), con la hipótesis de que el tipo de sedación puede tener un impacto en la supervivencia y otros resultados clínicamente relevantes.
DiseñoRevisión sistemática y metaanálisis de ensayos aleatorizados y no aleatorizados.
ÁmbitoUCI.
PacientesSe incluyeron críticamente enfermos y postoperatorios.
IntervencionesNinguna.
Mediciones y resultados principalesLos estudios que comparaban los AV vs. los anestésicos iv utilizados en la UCI se buscaron de forma independiente y sistemática. Finalmente, se incluyeron 15 estudios (1.520 pacientes de perfil predominantemente quirúrgico necesitaron sedación de AV durante menos de 96h). El AV no tuvo impacto en la mortalidad por cualquier causa (calidad de los datos probatorios muy baja, Odds Ratio=0,82 [0,60-1,12], p=0,20). Sin embargo, el AV se asoció con una reducción de la duración de la ventilación mecánica (p=0,03) y aumento de los días sin ventilación mecánica (p<0,001). La AV también redujo los niveles postoperatorios de troponina cardíaca (24 horas), el tiempo hasta la extubación (p<0,001) y el despertar (p=0,04).
ConclusionesEn este metaanálisis, la sedación volátil vs. propofol causó el aumento de los días sin ventilación, la reducción de la duración de la ventilación mecánica, el tiempo hasta la extubación y la liberación de troponina en pacientes de la UCI médica o quirúrgica, mientras que en pacientes de la UCI quirúrgica el tiempo hasta el despertar se acortó.
Sedation, ‘the act of calming patients by the administration of sedative medications’,1 is frequently used in intensive care units (ICUs) to prevent arouse and delirium associated harm, relieve anxiety, and reduce the stress of being mechanically ventilated.2 Since agitation and anxiety occurs in about 30%–80% of patients being treated in ICU settings,1 sedation is a highly sought strategy for ICU patients.
Volatile anesthetics (VA) sedation uses isoflurane or sevoflurane to achieve the desired level of sedation.3 It is a more recent and a less frequently used strategy than the traditional intravenous strategy in the ICU setting.4 However, with the adoption of user friendly devices for VA delivery – AnaConDa (Sedana Medical, Danderyd Sweden) and MIRUS (Pall Medical, Dreieich, Germany) – VA have become increasingly popular among ICU practitioners.5,6 There are potential lungs protective properties7,8 and anti-inflammatory activity9,10 coupled with the intended endothelium-saving effect,11,12 that contribute to an increased use of VA in the ICU. Malignant hyperthermia and possible environmental pollution are the most common drawbacks of using VA.13,14
Early studies confirmed safety and feasibility of VA in the ICU settings15,16 and a meta-analysis of randomized trials found a significant reduction in time to extubation17 with findings confirmed in more recent systematic reviews.18
The aim of this systematic review and meta-analysis was to comprehensively assess published randomized and non-randomized peer-reviewed studies which compared VA and i/v anesthetics for ICU sedation, with the hypothesis that the type of sedation may have an impact on mortality and other clinically relevant outcomes.
MethodsThis study was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.19 Meta-analysis is registered in PROSPERO (ID: CRD42021277313).
Search strategyA systematic search of studies published over the past 10 years (2011–2021) was carried out in PubMed, Medline, Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, Google Scholar and the Russian Science Citation Index (RSCI) by four independent researchers (NE, LB, MY and KK). The search was carried out in the form of queries with details available in the supplemental material (Supplemental appendix 1). Additionally, the authors used the backward snowballing method (analysis of references of included articles and retrieved reviews) for further studies. We did not restrict search by language. Medical Subject Headings (MeSH) terms were applied.
Study selectionThe links obtained from the database were first independently examined at the title/annotation level by two researchers (NE and LB). Randomized controlled trials, prospective and retrospective cohort studies comparing inhaled (volatile) versus i/v sedation in the intensive care unit were considered. After removing duplicates, the appropriate publications were selected. The final decision on inclusion in this study was made based on the analysis of full-text articles. Divergences were resolved by consensus.
For this study, the following inclusion criteria were used: adult patients (≥18 years) who underwent inhalation or i/v sedation in the intensive care unit (no restrictions on dose or time of administration). The exclusion criteria were mixed groups (both inhaled and i/v sedation were used), small sample size (less than 25 patients in two groups), duplicate publications, animal studies, clinical guidelines, articles without a comparison group.
Outcome measures and data extractionBasic information about the study (design, sample size, intervention plan, inclusion criteria), information about the study objects (age, gender, underlying disease and surgery), types of anesthetics and doses, target sedation level, anesthetic-conserving device, ICU treatment outcomes (mortality, duration of mechanical ventilation, troponin level at 1 postoperative day, ventilator-free days, time to extubation, awakening time, length of stay (LOS) in ICU, hospital LOS, catecholamine requirements) was collected. We have not considered the type of anesthetic used in the surgery room. The data were independently extracted by two researchers (NE and MY) and subsequently compared with each other for verification. The primary outcome of this study was all-cause mortality (30-day mortality). Secondary endpoints were: length of stay in ICU and length of hospitalization (days), duration of mechanical ventilation (days) and ventilator-free days, catecholamine requirements, cardiac troponin levels on the first postoperative day, time to extubation (min), and awakening time (min).
Internal validity and risk of bias assessmentThe internal validity and risk of bias of the included studies were assessed by two peer reviewers (MY and LB) and peer-reviewed by a third (VL) according to the latest version of the “ROB 2” (the Cochrane tool for assessing risk of bias in randomized trials) and “ROBINS-I” (Risk Of Bias In Non-randomized Studies of Interventions) tools.20,21 Discrepancies in assessment were resolved by consensus. Publication bias was assessed using the Egger's test (MedCalc Statistical Software, version 19.5.6),22,23 and also by visual examination of the “funnel plot” charts. We also used a GRADE systematic approach to rate the certainty of evidence.24 Two review authors (MY and LB) worked independently to assess the quality of evidence, disagreements were resolved by consensus.
Data analysis and synthesisTo calculate and visualize the results of the meta-analysis in forest plots, the Cochrane tool “RevMan, version 5.3” was used. The heterogeneity of studies was assessed using the Cochran's Q test, and the degree of statistical agreement was measured using the coefficient of heterogeneity I2. The quantitative results of individual studies were brought to the form “mean±standard deviation” and the standardized difference of mean values (SMD) and its 95% confidence interval were calculated. Since many quantitative parameters in this meta-analysis a priori have a deeply skewed distribution, we used the log transformation for summary data of ventilator-free days, time to extubation, time to awakening and ICU/hospital length of stay.25 We used Cochrane handbook recommendations to re-express SMDs using rules of thumb for effect sizes (<0.40=small effect, 0.40–0.70=moderate effect, >0.70=large effect).20 Binary research results were used to calculate the odds ratio with the corresponding 95% confidence interval (CI) using the inverse variance method (Mantel–Haenszel method). For a pooled estimate of the magnitude of the standardized mean difference, two models were used: a fixed-effects model (in the case of low statistical inconsistency, I2<50%) and a random-effects model (I2≥50% and/or p<0.05). Statistical significance was set at 0.05 for hypothesis testing.
Sensitivity analysisSensitivity analyses were performed by considering additional subgroups – analyzing only studies with a low or moderate risk of bias (Hi-QOL studies), only RCT/non-randomized studies and by sequentially removing each study and reanalyzing the remaining data set (producing a new analysis for each study removed). Additionally, a subgroup of studies which used a prolonged sedation (>12h) regimen was evaluated.
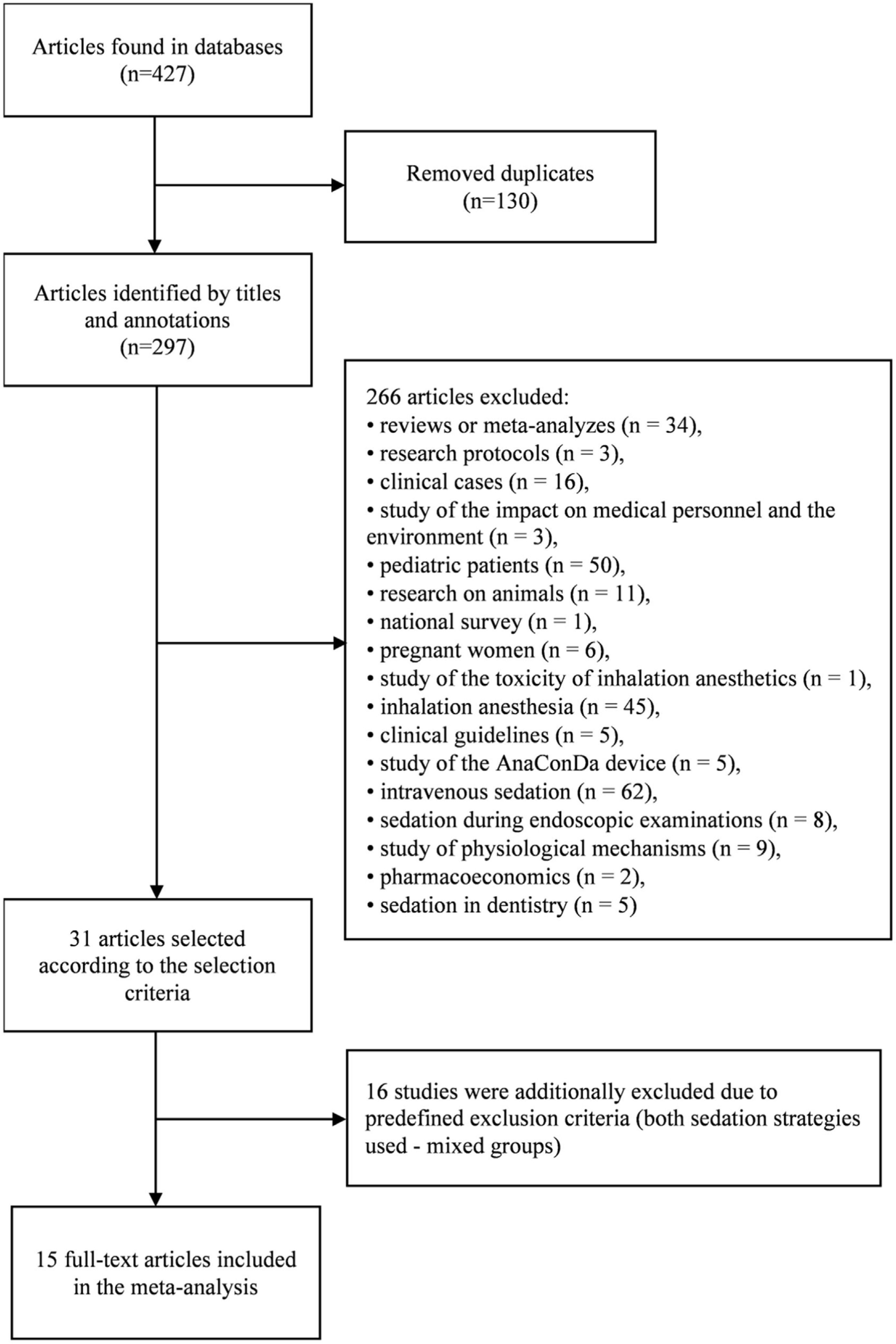
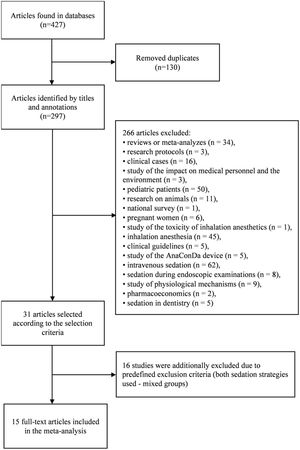
ResultsStudy characteristicsDuring the initial search, 427 articles were found, of which 31 were eligible. Upon careful reading of the full-text articles, 16 studies were excluded (Supplemental Table 1). Ultimately, 15 full-text articles published between January 2011 and July 2021were included in the meta-analysis. A flowchart illustrating the study selection process is presented in Fig. 1.
The characteristics of the included studies are summarized in Table 1.
Characteristics and description of the 15 trials included in the meta-analysis.
| Study | Design | N, VA | N, i/v | Volatile agent (dose) | Location volatile started | Anesthetic-conserving device | Comparator (dose) | Journal | Population |
|---|---|---|---|---|---|---|---|---|---|
| Bellgardt M et al. (2016)26 | Retrospective cohort | 72 | 128 | Isofluran MAC 0.3–0.8 | Operating room | AnaConDa | Propofol 2–4mg/kg/h; midazolam 0.05–0.2 mg/kg/h | Eur J Anaesthesiol | Postoperative surgical patients |
| Guerrero Orriach JL et al. (2013)27 | RCT | 20 | 40 | Sevoflurane MAC 0.5–0.7 | Operating room | AnaConDa | Propofol TCI 1–1.5mg/ml | J Crit Care | Postoperative cardiac surgery patients |
| Guinot PG et al. (2020)28 | RCT | 42 | 39 | Sevoflurane | Operating room | MIRUS | Propofol | Medicine | Postoperative cardiac surgery patients |
| Hellström J et al. (2012)29 | RCT | 50 | 50 | Sevoflurane ET 0.5–1.0% | ICU | AnaConDa | Propofol started at 2mg/kg/h | Scand Cardiovasc J | Postoperative cardiac surgery patients |
| Jabaudon M et al. (2017)30 | RCT | 25 | 25 | Sevoflurane started at 6ml/h | ICU | AnaConDa | Midazolam started at 0.1mg/kg/h | Am J Respir Crit Care Med | Adult patients with ARDS |
| Jerath A et al. (2015)30 | RCT | 67 | 74 | Sevoflurane or isofluran MAC 0.1–0.3 | Operating room | AnaConDa | Propofol 0.6–1.5mg/kg/h | Crit. Care Med | Postoperative cardiac surgery patients |
| Jung S et al. (2020)32 | Retrospective observational | 25 | 24 | Sevoflurane ET 0.5% | ICU | AnaConDa | Propofol 3.66±1.30μg/kg/h | Acute Crit Care | Postoperative surgical patients |
| Krannich А et al. (2017)33 | Retrospective observational propensity-matched | 110 | 110 | Isofluran ET 0.5–1.5% | ICU | AnaConDa | Midazolam 0.03–0.2mg/kg/h | Crit Care Med | Adult patients with non-traumatic cardiac arrest |
| Marcos-Vidal JM et al. (2014)34 | Prospective cohort | 67 | 62 | Sevoflurane ET 0.5–1.0% | ICU | AnaConDa | Propofol 1–4mg/kg/h | Heart Lung Vessel | Postoperative cardiac surgery patients |
| Meiser A et al. (2017)35 | Retrospective case match | 19 | 19 | Isofluran 3–10ml/h | ICU | AnaConDa | Propofol. midazolam | Respir Care | Adult patients with ARDS |
| Mesnil M et al. (2011)36 | RCT | 19 | 28 | Sevoflurane ET 0.5% | ICU | AnaConDa | Propofol started at 2mg/kg/h | Intensive Care Med | Adult patients, more than 24h of sedation for mechanical ventilation |
| Plotnikov GP et al. (2014)37 | RCT | 20 | 58 | Sevoflurane 5.0±2.3ml/h | ICU | AnaConDa | Propofol 4.35±1.75mg/kg/h. dexmedetomidin 0.35±0.2mg/kg/h | Byulleten’ NTSSSKH im. A.N. Bakuleva RAMN | Postoperative cardiac surgery patients |
| Resepov NА et al. (2017)38 | RCT | 20 | 20 | Sevoflurane started at 2ml/h (max 5ml/h) | ICU | AnaConDa | Propofol started at 0.5mg/kg/h | Messenger of Anesthesiology and Resuscitation | Adult patients with sepsis-associated delirium |
| Soro M et al. (2012)39 | RCT | 36 | 37 | Sevoflurane ET 0.5–1.0% | Operating room | AnaConDa | Propofol 1–4mg/kg/h | Eur J Anaesthesiol | Postoperative cardiac surgery patients |
| Staudacher DL et al. (2018)40 | Retrospective observational propensity-matched | 36 | 178 | Isofluran ET 0.5–1.0% | ICU | AnaConDa | Propofol | J Crit Care | Adult patients after cardiopulmonary resuscitation |
Abbreviations: VA: volatile anesthetics, i/v: intravenous anesthetics, ICU: intensive care unit, MAC: minimum alveolar concentration, RCT: randomized controlled trial, ET: end-tidal, TCI: target-controlled infusion.
The meta-analysis included 1520 patients: VA (628 patients); i/v sedation (892 patients). Among the 15 included studies, 9 were RCTs. The following settings were represented: seven studies in sedation in postoperative cardiac surgery patients,27–29,31,34,37,39 two articles in postoperative surgical patients,26,32 one study in patients who were assigned to more than 24h of mechanical ventilation sedation,36 five studies in the intensive care unit critically ill patients (non-traumatic cardiac arrest and successful CPR,33,40 sepsis-associated delirium38 and moderate to severe ARDS30,35).
Fourteen included studies used the AnaConDa device in the ICU for inhaled sedation, and only one study used MIRUS.28 Among the 15 included studies, 10 compared sevoflurane with propofol, 4 compared isoflurane with propofol and midazolam, and one study had multiple comparisons.
Four studies30,32,36,38 used long-term sedation (12h or more). Five of 9 RCTs and two of six non-randomized studies had medium or high quality (Supplemental Figures 1 and 2).
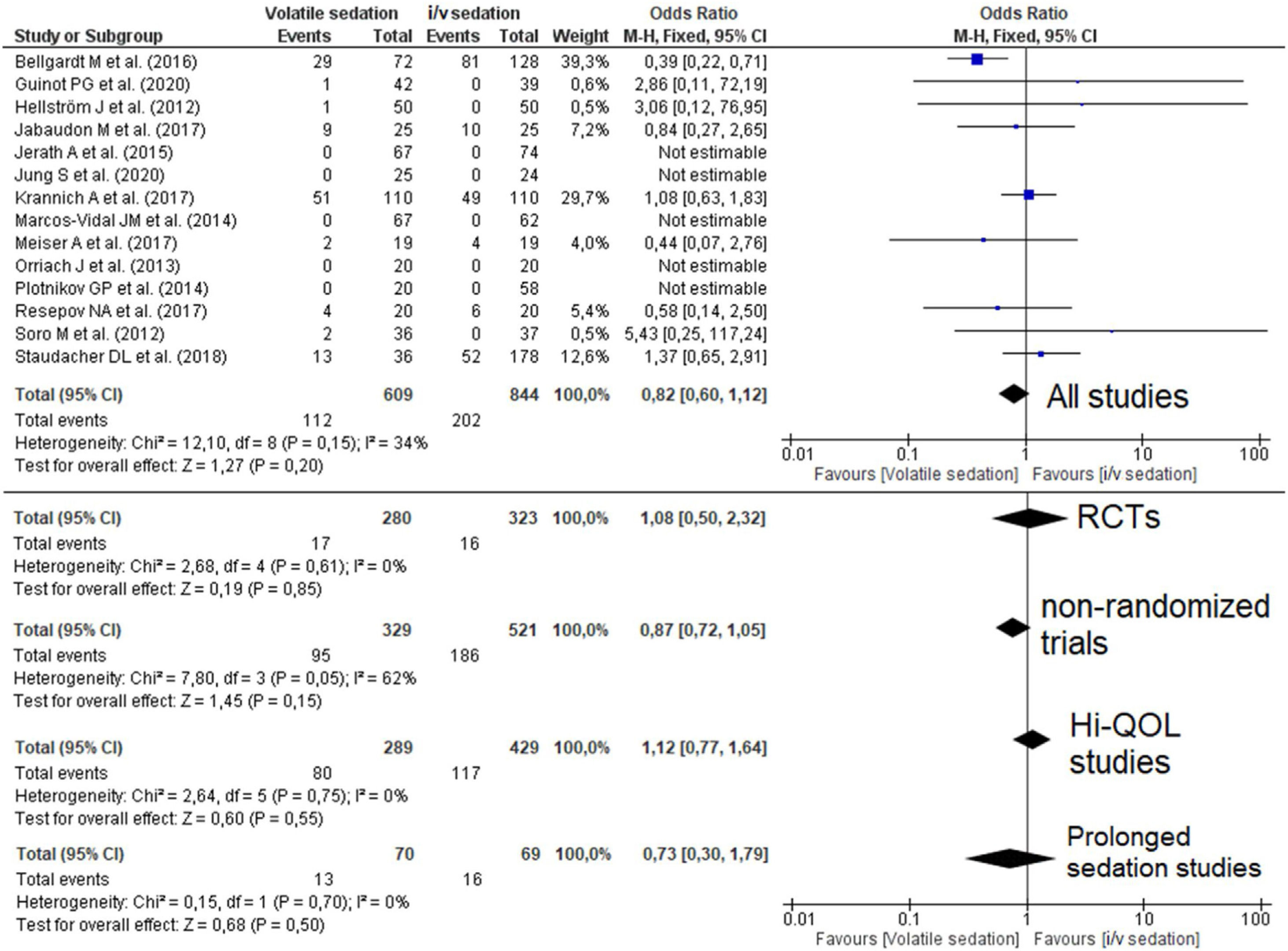
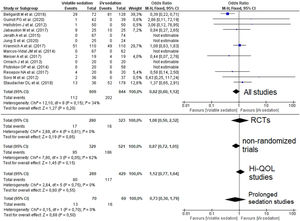
Quantitative data synthesisFourteen studies (8 RCTs) including 1453 patients reported mortality data which were not different between groups 112/609 (18.4%) in the VA group versus 202/844 (23.9%) in the i/v group (Odds Ratio (OR)=0.82 [0.60–1.12]; p-value for effect is 0.20; p-value for heterogeneity=0.15; I2=34%, Fig. 2; Table 2).
Forest plot for hospital mortality representing the odd's ratio for volatile vs. intravenous sedation effects on all-cause mortality for the included studies. The plot displays the study, sample size, odds ratio (OR), confidence interval (CI), and p-value. The size of the squares indicates the weight of the studies (taking into account sample size and standard deviations); the diamond represents the pooled OR with CI. Hi-QOL – studies with low–moderate risk of bias.
Outcomes and sensitivity analysis.
| Outcome | Trials | N, VA | N, i/v | SMD/OR | 95% CI | p-value for overall effect | p-value for heterogeneity | I2, % | p-value for publication bias |
|---|---|---|---|---|---|---|---|---|---|
| MortalityS | |||||||||
| All studies | 14 | 609 | 844 | 0.82 | 0.60–1.12 | 0.20 | 0.15 | 34 | p=0.19 |
| RCTs | 8 | 280 | 323 | 1.08 | 0.50–2.32 | 0.85 | 0.61 | 0 | p=0.02 |
| Non-randomized trials | 6 | 329 | 521 | 0.87 | 0.72–1.05 | 0.15 | 0.05 | 62 | p=0.86 |
| Studies with low–moderate bias | 7 | 289 | 429 | 1.12 | 0.77–1.64 | 0.55 | 0.75 | 0 | p=0.60 |
| Prolonged sedation | 3 | 70 | 69 | 0.73 | 0.30–1.79 | 0.50 | 0.70 | 0 | p=0.001 |
| Duration of mechanical ventilation | |||||||||
| All studies | 9 | 371 | 616 | −0.46 | −0.88–(−0.04) | 0.03* | <0.001 | 88 | p=0.49 |
| RCTs | 5 | 134 | 181 | −0.60 | −1.31–0.10 | 0.09 | <0.001 | 88 | p=0.85 |
| Non-randomized trials | 4 | 237 | 435 | −0.31 | −0.87–0.26 | 0.29 | <0.001 | 90 | p=0.86 |
| Studies with low–moderate bias | 5 | 191 | 333 | −0.52 | −0.72–(−0.33) | <0.001* | 0.09 | 54 | p=0.44 |
| Prolonged sedation | 3 | 64 | 73 | −0.11 | −0.45–0.22 | 0.51 | 0.70 | 0 | p=0.31 |
| Troponin level at 1 postoperative day | |||||||||
| All studies | 4 | 179 | 171 | −0.52 | −0.84–(−0.20) | 0.001* | 0.10 | 52 | p=0.49 |
| RCTs | 3 | 112 | 109 | −0.50 | −0.98–(−0.03) | 0.04* | 0.06 | 65 | p=0.35 |
| Studies with low–moderate bias | 2 | 62 | 59 | −0.61 | −1.56–0.34 | 0.21 | 0.02 | 82 | p<0.001 |
| Ventilator-free days | |||||||||
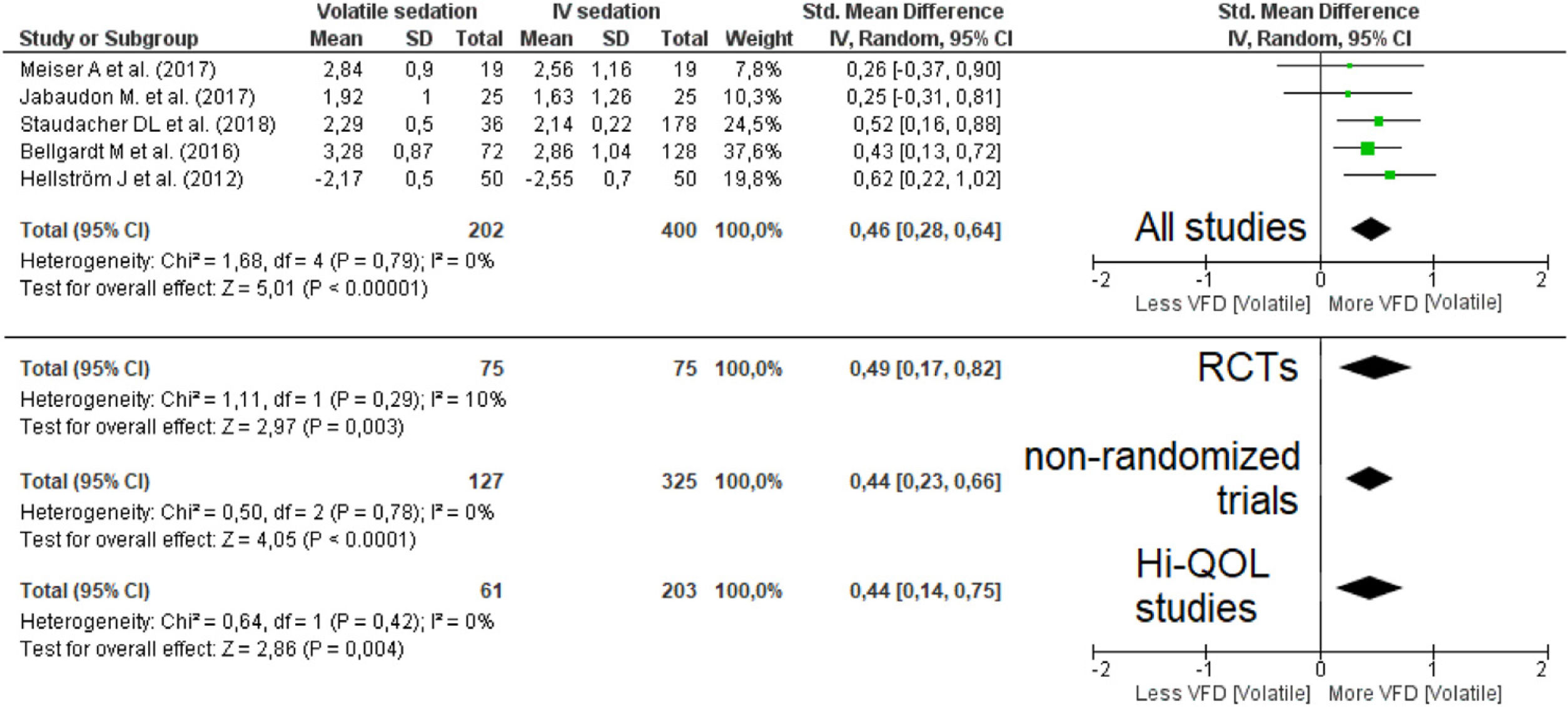
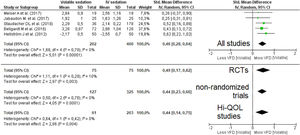
| All studies | 5 | 202 | 400 | 0.46 | 0.28–0.64 | <0.001* | 0.79 | 0 | p=0.40 |
| RCTs | 2 | 75 | 75 | 0.49 | 0.17–0.82 | 0.003* | 0.29 | 10 | p<0.001 |
| Non-randomized trials | 3 | 127 | 325 | 0.44 | 0.23–0.66 | <0.001* | 0.78 | 0 | p=0.59 |
| Studies with low–moderate bias | 2 | 61 | 203 | 0.44 | 0.14–0.75 | 0.004* | 0.42 | 0 | p<0.001 |
| Time to extubation | |||||||||
| All studies | 4 | 177 | 191 | −1.59 | −2.26–(−0.91) | <0.001* | <0.001 | 87 | p=0.06 |
| Awakening time | |||||||||
| All studies | 3 | 122 | 280 | −1.30 | −2.54–(−0.06) | 0.04* | <0.001 | 95 | p=0.41 |
| RCTs | 2 | 86 | 102 | −1.99 | −3.92–(−0.06) | 0.04* | <0.001 | 94 | p<0.001 |
| LOS in ICU | |||||||||
| All studies | 13 | 588 | 794 | 0.01 | −0.17–0.18 | 0.93 | 0.008 | 55 | p=0.88 |
| Hospital LOS | |||||||||
| All studies | 9 | 367 | 589 | −0.06 | −0.35–0.23 | 0.69 | <0.001 | 76 | p=0.03 |
| Catecholamine requirements | |||||||||
| All studies | 9 | 362 | 550 | 1.13 | 0.79–1.61 | 0.50 | 0.41 | 3 | p=0.43 |
Abbreviations: VA: volatile anesthetics, i/v: intravenous anesthetics, CI: confidence interval; ICU: intensive care unit; LOS: length of stay; RCT: randomized controlled trial; OR: odds ratio; SMD: standardized mean difference.
Analysis of subgroups of RCTs, non-randomized, Hi-QOL studies (studies with low–moderate risk of bias) and studies with long-term sedation did not reveal an association of sedation with mortality. Funnel plot for mortality is presented in Supplement (Supplemental Figure 3).
A pooled analysis of data from five studies (606 patients, four studies with short-term sedation, two RCTs) showed that patients on VA had an increase in ventilator-free days (moderate effect size: SMD=0.46 [0.28–0.64]; p-value for the effect <0.001; p-value for heterogeneity=0.79; I2=0%, Fig. 3; Table 2). The results were confirmed in all sub-analyses: RCTs (p=0.003), non-randomized (p<0.001) and Hi-QOL studies (2 trials, p=0.004).
Forest plot for ventilator-free days. The plot displays the study, sample size, log-transformed standardized mean difference (SMD), confidence interval (CI), and p-value. The size of the squares indicates the weight of the studies (taking into account sample size and standard deviations); the diamond represents the pooled SMD with CI. Hi-QOL – studies with low–moderate risk of bias.
A pooled analysis of 987 patients (9 studies, 5 RCTs) showed that sedation with VA was associated with a reduction in duration of mechanical ventilation (SMD=−0.46 [−0.88 to −0.04] – moderate effect size; p-value for effect=0.03; p-value for heterogeneity <0.001; I2=88%, Supplemental Figure 4, Table 2). The results were confirmed in Hi-QOL studies (5 trials, p<0.001), but not when considering only RCTs (p=0.09) or non-randomized studies (p=0.29) (Table 2). When considering only studies using prolonged sedation there was also no significant effect (p=0.51).
Four studies with short-term sedation using sevoflurane (3 RCTs, 350 patients) reported cardiac troponin levels (1 day after surgery) with postoperative cardiac surgery patients on VA having a statistically significantly lower troponin levels (moderate effect size: SMD=−0.52 [from −0.84 to −0.20]; p-value for effect 0.001; p-value for heterogeneity=0.10; I2=52%, Supplemental Figure 5; Table 2). The result was robust when considering RCTs (p=0.04) but not Hi-QOL studies (2 trials, p=0.21).
Data from 4 studies (all RCTs, 3 with short-term sedation) in 368 patients showed that sedation with VA was associated with a decrease in time to extubation in both medical and surgical patients (large effect size: SMD=−1.59 [−2.26 to −0.91]; p-value for effect <0.001; p-value for heterogeneity <0.001; I2=87%, Supplemental Figure 6; Table 2). Results on awakening time (large effect size: SMD=−1.30 [−2.54 to −0.06]; p-value for effect 0.04; p-value for heterogeneity <0.001; I2=95%) were confirmed when non-randomized studies were excluded (p=0.04, Supplemental Figure 7 and Table 2). The difference in the awakening time was established only for surgical patients, not for medical ones. However, only one study was of high quality. Moreover, the time to extubation and the awakening time were less in VA group than in i\v group, regardless of the duration of sedation (p<0.05). Despite the depth of sedation across the majority of the studies were approximately similar (from RASS=−1 to RASS=−3), these results were difficult to compare them to the ones in the other studies where the depth of sedation were measured according to other scales.
No differences were observed in length of ICU stay (p=0.93), length of hospital stay (p=0.69), and need for catecholamines (p=0.5, Table 2 and Supplemental Figures 8–10).
Certainty of evidence for all studied outcomes was qualified using GRADE approach for RCTs. Overall, very low quality of evidence shows that volatile sedation has no impact on hospital mortality. Certainty of evidence for other outcomes ranged from very low to high. The reasons for the decrease in the quality of evidence are summarized and presented in Table 3.
Certainty of evidence from RCTs for studied outcomes (Grade approach).
| Outcome | No. of participants and RCTs | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Upgrades | Overall quality of evidence |
|---|---|---|---|---|---|---|---|---|
| Mortality statement | ||||||||
| No effect on ICU patients | 525, 7 RCTs | Not serious (0) | Serious (−1) | Not serious (0) | Very serious (−2) | Serious (−1) | None | ⊕OOOVery low |
| Duration of mechanical ventilation (DMV) statement | ||||||||
| Volatile anesthetics do not reduce DMV in ICU patients | 315, 5 RCTs | Serious (−1) | Very serious (−2) | Not serious (0) | Very serious (−2) | Not serious (0) | None | ⊕OOOVery low |
| Troponin level at 1 p/o day statement | ||||||||
| Volatile anesthetics reduce troponin level after cardiac surgery | 221, 3 RCTs | Not serious (0) | Serious (−1) | Not serious (0) | Serious (−1) | Not serious (0) | None | ⊕⊕OOLow |
| Ventilator-free days (VFD) statement | ||||||||
| Volatile anesthetics increase VFD in ICU patients | 150, 2 RCTs | Serious (−1) | Very serious (−2) | Not serious (0) | Not serious (0) | Serious (−1) | None | ⊕OOOVery low |
| Time to extubation (TE) Statement | ||||||||
| Volatile anesthetics reduce TE in ICU patients | 368, 4 RCTs | Serious (−1) | Not serious (0) | Not serious (0) | Not serious (0) | Not serious (0) | None | ⊕⊕⊕OModerate |
| Awakening time (AT) statement | ||||||||
| Volatile anesthetics reduce AT in ICU patients | 188, 2 RCTs | Serious (−1) | Serious (−1) | Not serious (0) | Serious (−1) | Not serious (0) | None | ⊕OOOVery low |
| LOS in ICU statement | ||||||||
| No effect on ICU patients | 532, 7 RCTs | Not serious (0) | Serious(−1) | Not serious (0) | Not serious (0) | Not serious (0) | None | ⊕⊕⊕OModerate |
| Hospital LOS statement | ||||||||
| No effect after cardiac surgery | 455, 5 RCTs | Not serious (0) | Serious (−1) | Not serious (0) | Serious (−1) | Not serious (0) | None | ⊕⊕OOLow |
| Catecholamine requirements statement | ||||||||
| No effect on ICU patients | 520, 6 RCTs | Serious (−1) | Not serious (0) | Serious (−1) | Serious (−1) | Not serious (0) | None | ⊕OOOVery low |
Abbreviations: RCT, randomized controlled trial; p/o, postoperative; ICU, intensive care unit; LOS, length of stay.
Key: 0, no evidence downgrade; −1, serious limitation; −2, very serious limitation; +1, evidence upgrade. Baseline evidence level for RCTs: high.
Overall analysis showed that the use of VA for sedation in patients in the ICU does not affect hospital mortality (OR=0.82 [0.60–1.12]; p=0.20). This finding is consistent with previous meta-analyses.17,41 The level of evidence obtained in current study for mortality outcome was downgraded to very low due to clinical inconsistency, presence of publication bias and serious imprecision (wide confidence interval for overall effect). It should be noted that the presence of publication bias was found, and statistical heterogeneity was not high (I2=34%).
In this study, inhalation sedation in the ICU was associated with an increase in ventilator-free days, but the final level of evidence was very low. To the best of the authors’ knowledge, this was the first meta-analysis which has shown impact of VA on patients’ ventilator-free days.
This meta-analysis performed mostly from studies including surgical patients suggests that volatile sedation is associated with a reduction in duration of mechanical ventilation. In a meta-analysis by Jerath et al. (2017)41 (523 patients, 8 studies) the duration of mechanical ventilation was lower in VA group (p=0.03).
According to our data, there is moderate quality evidence that VA reduce time to extubation in ICU patients and very low quality evidence that VA reduce time to awakening (when including only RCTs). In the meta-analysis of Landoni et al. (2016) the use of halogenated agents was associated with a significant reduction in time to extubation (p<0.00001).17 Meta-analysis of Kim et al. (13 studies, 1027 patients) showed that volatile sedation delivered through a special AnaConDa device in the ICU shortens the awakening (p=0.004) and extubation time (p<0.001) compared to i/v anesthetics sedation.4 A similar result was obtained in a meta-analysis by Jerath et al. (2017),41 which also showed a decrease in extubation time when using inhaled sedation compared with i/v sedation (p<0.00001).
Halogenated anesthetics are known to have cardioprotective effects,26,42 which, according to our study, was expressed in a decrease in the content of specific markers – cardiac troponins on the first postoperative day in postoperative cardiac surgery patients. This fact has also been confirmed by a number of other studies.43,44 Results from a meta-analysis by Kim et al. (2017) also indicate a decrease in troponin levels in patients with VA, and the effect size was largest between 12 and 24hours after ICU admission (p=0.003).4 Patients in VA group had 0.71ngml−1 (95% CI: 0.23 to 1.2) lower troponin levels according to a meta-analysis by Spence et al. (2017).45
According to the results of our research there was no impact of VA on catecholamine requirements, which was confirmed in the sensitivity analysis and in the review of studies with prolonged sedation, however the evidence was of very low quality. The impact of VA and i/v anesthetics on this outcome has not been assessed in other meta-analyses to date.
No relationship was found between the type of sedation and length of ICU stay and in hospital LOS in current meta-analysis. This fact has also been confirmed in other studies. Thus, in a meta-analysis of Landoni et al. (2016) no relationship was found between VA and ICU LOS (p=0.13) and hospital LOS (p=0.08).17 Similar results were obtained in meta-analyses of Jerath et al. (hospital LOS: p=0.74), Kim et al. (ICU LOS: p=0.513; hospital LOS: p=0.059) and Spence et al. (ICU LOS: p=0.65; hospital LOS: p=0.11).4,41,45
We were the first to use the GRADE approach to assess the quality of the evidence regarding the effect of sedation on mortality. Increase of ventilator free days due to volatile sedation firstly depicted in current meta-analyses and it seems to be a new and important piece of information that have been brought to our knowledge. It looks like that the next strength of our meta-analysis is that we performed very thorough sensitivity analyses, looking at both RCT alone and low-risk of bias studies.
Authors acknowledge that this study also has some limitations. All RCTs included in the meta-analysis were single center studies and therefore external validity is limited. The meta-analysis was performed mostly from studies included surgical patients so the cohort of medical critically ill patients is poorly represented. The sample sizes of the included studies were small and only four studies used long-term sedation (12h or longer). Clinical heterogeneity (inconsistency) of studies (mixed surgery, varying depth of sedation, different time points for measuring outcomes) and risks of bias also reduced the level of evidence. We failed to spot any difference in effect on the investigated parameters between isoflurane and sevoflurane been used for inhaled sedation. Thus, some of the results may have been insufficient. We also have not considered the type of anesthetic used in the surgery room. Finally, we were unable to analyze other factors such as laboratory parameters, cognitive status and major morbidity as most of the included studies did not report these data.
ConclusionVolatile anesthetics had no effect on hospital mortality (very low evidence), however they reduced duration of mechanical ventilation, troponin level, time to extubation, and the time to awakening in medical and surgical ICU patients. Thus, it seems like, despite of new data, brought by current meta-analysis, further large high-quality randomized controlled studies are to be performed to provide more knowledge towards this still unclear challenge due to the low power of published studies.
Author contributionsConceived and designed the análisis: VL, GL, NE, MY, LB, KK, OG, AK
Collected the data: NE, MY, LB, KK
Contributed data or analysis tools: GL
Performed the analysis: MY, KK
Wrote the paper: VL, GL, NE, MY, LB, KK, OG, LO, AK
All authors have approved the final article.
FundingNone.
Conflict of interestNone.