Cardiogenic shock (CS) is characterized by the presence of a state of tissue hypoperfusion secondary to ventricular dysfunction. Hemodynamic monitoring allows us to obtain information about cardiovascular pathophysiology that will help us make the diagnosis and guide therapy in CS situations. The most used monitoring system in CS is the pulmonary artery catheter since it provides key hemodynamic variables in CS, such as cardiac output, pulmonary artery pressure, and pulmonary artery occlusion pressure. On the other hand, echocardiography makes it possible to obtain, at the bedside, anatomical and hemodynamic data that complement the information obtained through continuous monitoring devices.
CS monitoring can be considered multimodal and integrative by including hemodynamic, metabolic, and echocardiographic parameters that allow describing the characteristics of CS and guiding therapeutic interventions during hemodynamic resuscitation.
El shock cardiogénico (SC) se caracteriza por la presencia de un estado de hipoperfusión tisular secundario a disfunción ventricular. La monitorización hemodinámica nos permite obtener información acerca de la fisiopatología cardiovascular que nos ayudará a realizar el diagnóstico y guiar la terapéutica en las situaciones de SC. El sistema de monitorización más utilizado en el SC es el catéter de arteria pulmonar puesto que proporciona variables hemodinámicas clave en el SC, como son el gasto cardíaco, la presión de arteria pulmonar y la presión de oclusión de arteria pulmonar. Por otro lado, la ecocardiografía permite obtener, a pie de cama, datos anatómicos y hemodinámicos que complementan la información obtenida mediante los dispositivos de monitorización continua.
La monitorización del SC puede considerarse multimodal e integradora al incluir parámetros hemodinámicos, metabólicos y ecocardiográficos que permiten describir las características del SC y guiar las intervenciones terapéuticas durante la reanimación hemodinámica.
From the pathophysiological perspective, the primary phenomenon characterizing cardiogenic shock (CS) is ventricular dysfunction with a low cardiac output (CO) that leads to tissue hypoperfusion and multiorgan failure.1,2 Other alterations in turn add to cardiac dysfunction, such as inflammation, ischemia and vascular tone disorders.2 The complexity of the clinical and hemodynamic manifestations of CS is a consequence of these pathophysiological components, the etiology and underlying mechanisms, the severity of shock, and the type of ventricular alteration involved.1–5
Hemodynamic monitoring is of great help in the resuscitation of patients with CS, since it affords hemodynamic data that contribute to describing the characteristics and severity of shock. In addition, it can precisely detect and monitor organ dysfunction and tissue oxygenation, and serves as a guide for optimizing the use of vasopressors and inotropic agents. Likewise, hemodynamic monitoring provides information for deciding the introduction of mechanical support. It is important to note that while monitoring is particularly useful in the early phases of hemodynamic resuscitation, it is of less utility once organ failure has become established.4,5
The international recommendations emphasize the need to start basic hemodynamic monitoring in the first hours of shock. On the other hand, in patients with an insufficient response to the measures applied in the first 3–6 hours, or in individuals with complex shock, where greater pathophysiological knowledge of the process may be required, a greater degree of continuous hemodynamic monitoring can be considered1,4,5 in order to optimize our interventions, quantify their effects, and avoid complications derived from the applied treatments. Echocardiography likewise affords anatomic and hemodynamic information capable of complementing the data obtained from continuous monitoring devices.
Basic hemodynamic monitoringArterial pressureThe measurement of arterial pressure (AP) is one of the most classical methods for monitoring organ perfusion.6 In the critically ill patient, it is advisable to perform invasive monitoring to ensure more reliable and precise measurements. The monitoring of AP also allows us to determine pulse pressure variation (PPV), though its usefulness and that of other dynamic volume response parameters in CS have not been clearly established.7
The minimum pressure values needed to maintain self-regulation in patients with CS have not been fully defined, and the recommendation to reach a mean blood pressure (MBP) of 65 mmHg comes from the extrapolation of data obtained from other types of shock. Some studies report that organ dysfunction probably begins with MBP < 75–80 mmHg.6,8 In the same line, other studies of patients presenting CS of ischemic origin have shown an increase in MBP to 80 mmHg to be associated with a better cardiac index (CI) and improved central venous saturation (ScvO2) values.9 In this regard, a study found that patients presenting MBP 85–100 mmHg in the first 24 hours of CS of ischemic origin exhibited better survival figures than those with MBP < 65 mmHg.10 Nevertheless, other studies have reported no improvement in perfusion with an increase in MBP induced by the use of noradrenaline.11 In the absence of clear evidence on the target MBP value, it seems reasonable to reach MBP 65–70 mmHg.
Central venous pressureCentral venous pressure (CVP) is a complex variable that reflects right atrial pressure (RAP) as well as right ventricle (RV) preload, and can be used to estimate the degree of extrathoracic organ congestion. Although its reliability in representing patient volume response is subject to controversy, CVP offers interesting information about the trends in patient volemia.12 Central venous pressure can be useful in distinguishing between organ failure related to congestion versus tissue hypoperfusion. In addition, it is an early indicator of RV dysfunction; the continuous measurement of CVP in patients with CS is therefore important.13 In fact, in patients with CS subjected to left ventricle (LV) mechanical support, CVP > 12 mmHg indicates RV dysfunction, which is associated with increased mortality.14
Peripheral perfusion and tissue oxygenation parameters- SvcO2 or mixed venous saturation (SvO2): These parameters reflect the relationship between oxygen demand and supply; a decrease in their values indicates insufficient availability.4 Cardiogenic shock is characterized by an increase in oxygen extraction by the cells, with a consequent decrease in the venous saturation values. These parameters may be altered before an increase in lactate levels is observed; they therefore allow the early identification of tissue hypoxia. Excessively high values may also indicate cell incapacity to extract oxygen, with cellular failure.6
- Lactate: This metabolite of anaerobic metabolism has a normal value of 1 mEq/l, and small increments have been associated with an increase in patient mortality. Such increments may be due to an increase in lactate production or to a decrease in lactate clearance.15 The dominant paradigm is that hyperlactatemia is secondary to the appearance of anaerobic glycolysis induced by tissue hypoperfusion. However, some studies suggest that the increase in lactate production would also be secondary to the increase in its aerobic production, independently of clearance, probably due to adrenergic stimulation.16 Thus, lactate elevation would be a consequence of both low output and hypoperfusion, and adrenergic stress.
The Society for Cardiovascular Angiography and Intervention (SCAI) classification of CS uses lactate as a key element in the identification and stratification of patients of this kind. There are even hospital protocols for the management of CS that use lactate, among other parameters, for guiding treatment.17
It must be taken into account that lactate levels are not static but evolve over time; it, therefore, has been suggested that the assessment of these changes over time could improve the prognostic capacity of lactate and afford more information than a mere point determination in time for guiding patient care.16,18
Several studies have explored the use of lactate as a CS prognostic and severity marker. In the sub-analysis of the IABP-SHOCK II trial carried out by Fuernau et al.,19 the lactate values recorded 8 hours after admission showed greater mortality prognostic capacity than the values recorded at baseline or clearance measured during the first 8 hours. In turn, a concentration cut-off value of 3.1 mEq/L recorded 8 hours after admission was identified as the best prognostic indicator in patients with CS.19 In a study derived from the DOREMI trial, lactate clearance was identified as a good prognostic marker and facilitated the guidance of treatment.20 In another study originating from the Cardshock trial, the authors concluded that the lactate levels recorded at baseline and after 6, 12 and 24 hours are predictive of mortality, in the same way as the decrease in lactate for 24 hours.15
- GapCO2: This tissue hypoperfusion parameter corresponds to the venous-arterial difference or gap in pCO2 (P(v-a)CO2).21,22 In situations of low CO, the anaerobic increase in CO2 production, together with venous stasis, produces an increase in GapCO2 difference; values > 6 mmHg are associated with tissue hypoperfusion.23,24 The scientific evidence in relation to patients with CS is scarce, however. A study in patients of this kind conducted to assess the GapCO2 kinetics found that the measurements made upon admission and after 6, 12, 24 and 48 hours were higher among the non-survivors than in the survivors, though statistical significance was not reached.21 Likewise, these authors found that GapCO2 < 6 mmHg recorded 12 hours after admission was able to identify those patients with a low risk of in-hospital mortality. On the other hand, they observed that patients with higher GapCO2 values presented refractory CS.21 In another study involving post-cardiotomy shock patients, the GapCO2 values were seen to be more closely related to the prognosis than SvcO2 or the lactate levels. Furthermore, a correlation was found between the cardiac index (CI) and GapCO2, leading the authors to suggest that the identification of patients with high GapCO2 values could help to identify those individuals with low CO.22 Studies in patients with CS and venous-arterial extracorporeal membrane oxygenation (ECMO) have reported improved survival figures in those patients with GapCO2 < 6 mmHg in the first 24 hours from implantation of the device.25
- Capillary refill time (CRT) and mottling: The evaluation of peripheral perfusion includes clinical signs such as CRT and lividness. These signs are easy to evaluate at the patient's bedside (“easy to use, easy to learn”), and appear to be related to the severity of organ dysfunction and the clinical course, independently of the systemic hemodynamics.26 It has been seen that these markers of peripheral perfusion, along with GapCO2, are associated with flow disorders at a microcirculatory level in septic shock.27 Several studies have shown that CS is also characterized by macro- and microcirculatory alterations; therefore, these markers could prove useful in this situation.26–28
Recent studies have evaluated these parameters in CS. The FRENSHOCK multicenter observational registry29 has evidenced the presence of lividness in 39% of the patients with CS at the time of admission. These subjects presented higher mortality rates after 30 days (31% versus 23%, p = 0.031) and at one year (54% versus 42%, p = 0.003) than the patients without lividness. On the other hand, Merdji et al.30 found CRT > 3 s to be associated with greater mortality at 90 days (hazard ratio [HR] 10.50, confidence interval: 2.48–45.3) in a population of patients with CS.
Advanced hemodynamic monitoringPulmonary artery catheterThe most widely used advanced hemodynamic monitoring system in CS is the pulmonary artery catheter (PAC). Its introduction by Swan and Ganz in 1970 represented a revolution in monitoring in Intensive Care Medicine. In effect, the PAC has improved knowledge of cardiovascular function in the critically ill patient, allowing the calculation of CO through thermodilution, as well as pulmonary artery pressure (PAP), pulmonary artery occlusion pressure (PAOP) and also oxygen transport/consumption parameters (DO2 and VO2).4,5 The PAC has been used to define CS (cardiac index < 2.2 l/min/m2 and PAOP > 15 mmHg in the case of left ventricle dysfunction) or to establish classical hemodynamic patterns (hypovolemia, acute lung edema, cardiogenic shock, obstructive shock, etc.).31 The recent classification of the SCAI32 describes 5 severity grades of CS that include different hemodynamic parameters (Fig. 1).
Hemodynamic characteristics of the Society for Cardiovascular Angiography and Intervention (SCAI) classification17 of cardiogenic shock.
CP: cardiac power; HR: heart rate; VF: ventricular fibrillation; CI: cardiac index; PAPI: pulmonary artery pulsatility index; MBP: mean blood pressure; SBP: systolic blood pressure; PAOP: pulmonary artery occlusion pressure; CVP: central venous pressure; SvO2: mixed venous oxygen saturation.
The PAC can be a useful tool for managing complex circulatory conditions in which knowledge of PAP, PAOP and tissue oxygenation parameters (e.g., acute right- and left-side failure, pulmonary hypertension, or patients subjected to heart surgery, with mechanical support, or pending heart transplantation) is considered to be particularly important.1,4,5,33
On the other hand, the PAC allows us to calculate other relevant hemodynamic parameters such as cardiac power (CP) and the pulmonary artery pulsatility index (PAPI) (Table 1):
- -
CP: The heart may be regarded as a mechanical pump capable of generating hydraulic energy that can be expressed as cardiac power, defined as the product of the flow and pressure generated by the heart. Thus, CP is the product of CO and MBP (CP = CO x MBP x 0.0022). Different studies have found CP to be associated with the prognosis of patients with CS.34–36 Specifically, CP ≤ 0.53 has been seen to be the greatest independent predictor of in-hospital mortality among patients with acute myocardial infarction (AMI complicated with CS in the SHOCK registry).35 Cardiac power can be recorded using any hemodynamic monitoring system that determines CO, or employing echocardiography.
- -
PAPI: This parameter reflects the components of the RV: venous system, RV function and pulmonary circulation relating to pulmonary artery pulse pressure and RAP. It is calculated from the formula: (systolic pulmonary artery pressure – diastolic pulmonary artery pressure)/RAP. The parameter can be used as a predictor of RV failure after left ventricular assist placement. In addition, PAPI has shown a high in-hospital mortality predictive capacity in patients with AMI of the RV.33,37,38
Hemodynamic parameters.
| CP: cardiac powerMBP x CO x 0.0022 N: >1 WSVRi: systemic vascular resistance index(MBP - CVP) x 80/CI N: 1800–2800 dynes.s.cm-5 m2PVRi: pulmonary vascular resistance index(PAPm - PAOP) x 80/CI N: 200–350 dynes.s.cm-5 m2PAPI: pulmonary artery pulsatility index (PAPs - PAPd)/RAP N: >2.0CFI: cardiac function indexCO/GEDV N: 4.5–6.5 l/minGEF: global ejection fractionSV/GEDV/4 N: 25–35% GEDVi: global end-diastolic volume index N: 680–800 ml/m2ELWi: extravascular lung water index N: 3–7 ml/kgPVPI: pulmonary vascular permeability index N: 1–3 |
CO: cardiac output; ELWi: extravascular lung water index; PVPI: pulmonary vascular permeability index; RAP: right atrial pressure; MBP: mean blood pressure; PAPd: diastolic pulmonary artery pressure, PAPm: mean pulmonary artery pressure; PAPs: systolic pulmonary artery pressure; PAOP: pulmonary artery occlusion pressure; CVP: central venous pressure; SV: systolic volume; GEDV: global end-diastolic volume.
The randomized, multicenter ESCAPE trial,39 published in 2005, included 433 patients presenting heart failure without CS, assigned to two therapeutic groups: one group guided by information obtained from PAC, and the other group guided only by the data afforded by clinical evaluation. In this patient population, no differences were found in terms of morbidity-mortality between the two groups. The results of this study generated even more uncertainty regarding the role of PAC in shock.
However, it is important to take into account that most of the initial studies that were designed to evaluate the impact of PAC upon the clinical evolution of the patients - including the ESCAPE trial - excluded individuals with CS. For this reason, the role of PAC in such patients remains unclear.40 Nevertheless, recent studies in patients with CS have shown the use of PAC to be associated with an improved clinical course.41–45
Specifically, the Cardiogenic Shock Working Group, a large multicenter registry representing the “real world” of the diagnostic and therapeutic strategy in patients with CS, found the availability of full hemodynamic data before the start of mechanical assist measures to be associated with greater survival in all the SCAI grades SCAI.41 This finding was consistent with the results of other studies such as that published by Tehrani et al.42 These authors evaluated a therapeutic strategy based on a “shock team”, and found that the algorithm using the information obtained from invasive hemodynamic monitoring, including a score with CP and PAPI, improved the patient outcomes. Likewise, other single or multicenter studies have also reported a decrease in short- and long-term mortality with the use of PAC in patients presenting CS.43,44 Lastly, a very recent meta-analysis including observational studies has reported a lower mortality rate with the use of PAC.45 All these promising results in favor of the use of PAC need to be confirmed by prospective multicenter trials. The international consensuses and guides recommend PAC in selected patients who fail to respond to initial treatment (IIB/C), or in the case of uncertainty regarding the diagnosis or therapy (cases of mixed shock or patients with severe RV dysfunction).5,46–48
Transpulmonary thermodilution and minimally invasive systemsOn the other hand, advanced hemodynamic monitoring devices based on transpulmonary thermodilution (TPTD) allow us to estimate CO (TPTD technique and via pulse wave analysis calibrated with TPTD). Furthermore, other parameters can also be obtained, such as the global end-diastolic volume index (GEDVi), cardiac function index (CFI), global ejection fraction (GEF), extravascular lung water (EVLW), and the pulmonary vascular permeability index (PVPI)33,49,50 (Table 1). A low CFI should alert us to a possible alteration of LV motility, though echocardiographic assessment is essential to discard RV dysfunction. On the other hand, TPTD cannot be used in patients with mechanical assist measures.33
In contrast, devices that calculate CO based on analysis of the pulse wave contour without external calibration should not be used in patients with CS, due to their poor reliability in situations characterized by low CO. 51 Finally, noninvasive monitoring systems such as bioreactance are not advised in this patient population, due to their lack of reliability and precision.33,52
Objectives of hemodynamic resuscitation in cardiogenic shockThe ultimate objective of hemodynamic resuscitation in any kind of shock is to restore the tissue oxygen supply in accordance with the metabolic requirements. In clinical practice, such resuscitation consists of optimizing CO to revert the clinical and metabolic signs of tissue hypoperfusion (lactate, venous oxygen saturation) and guarantee sufficient MBP to maintain the required minimum perfusion of the tissues.1,4
In CS, accepted management seeks to increase CO and reduce PAOP to under 15 mmHg. However, to date, no hemodynamic resuscitation algorithm has prospectively determined whether reaching concrete CO targets as a therapeutic goal can improve the prognosis of CS. In this regard, there is no clear evidence as to what the ideal “resuscitation target” parameters are in CS. Despite this lack of evidence, the data published in relation to the outcomes of CS management strategies, involving multidisciplinary teams (“shock teams”) and CS therapeutic protocols, offer information that can be very useful in decision-making.42,53,54,56,57
These strategies include the Inova Heart and Vascular Institute Cardiogenic Shock Initiative of Tehrani et al.,42 as commented above. The proposed resuscitation algorithm included the serial measurement of lactate, CP and PAPI, evidencing improved patient survival when securing CP > 0.6 W, lactate < 3 mg/dl and PAPI < 1.0. On the other hand, the National Cardiogenic Shock Initiative53–55 also included CP and PAPI in its therapeutic protocol. In a population of patients with CS secondary to AMI, this multicenter study found lactate < 4 mmol/l and CP > 0.6 W after 12–24 hours to be associated with an in-hospital survival rate of 95%. The authors therefore concluded that these parameters could guide early decision making in CS. Lastly, the UTAH Cardiac Recovery Shock Team56 proposed a hemodynamic resuscitation protocol in which treatment progression to a mechanical assist device was based on the persistence of signs of tissue hypoperfusion and/or CI < 2.2 l/min/m2 together with PAOP or LV end-diastolic pressure > 15 mmHg.
A very recent study has proposed a hemodynamic resuscitation algorithm in patients with CS secondary to AMI with “resuscitation targets” that include CP > 0.6 W, PAPI > 1, lactate < 4 mmol/l and the need for less than two vasopressor drugs.57 Those patients that reached the four target parameters after 24 hours showed improved in-hospital survival (odds ratio [OR] 11.21, confidence interval: 1.7–123.7).
At present it is unclear how CRT and the presence of lividness may be integrated under the “resuscitation targets” of CS.
Echocardiography in cardiogenic shockEchocardiography as a diagnostic tool is noninvasive, safe, available at the patient’s bedside, relatively inexpensive, and can be quickly performed and interpreted in acute situations. These characteristics define it as a first-line diagnostic and monitoring option in the differential evaluation of hemodynamic instability, and particularly in CS.1,58
In this regard, echocardiographic evaluation can be stratified, with a first exploration performed in the early stage of care,3 at the patient’s bedside,4 followed by an advanced echocardiographic exploration (transthoracic or transesophageal) by an expert with advanced skills.59
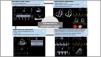
Basic echocardiographyThe initial echocardiographic exploration should be performed as soon as possible in the identification phase. It offers a basic evaluation of left and right ventricle contractility, supporting or discarding the diagnosis of CS. In addition, it can help to identify potentially fatal etiologies of shock such as cardiac tamponade (Fig. 2).5
Usefulness of echocardiography in cardiogenic shock.
Calculation of cardiac output based on the formula: systolic volume = area of the left ventricle outflow tract x velocity-time integral of the left ventricle outflow tract. Evaluation of left and right ventricular function, and analysis of filling pressures and pulmonary artery pressure based on pulsed doppler recording of transmitral flow in relation to tissue doppler mitral ring study.
The left ventricular ejection fraction (LVEF) is the parameter most widely used in clinical practice to assess left ventricle function. It quantifies the global systolic function of the LV, though it must be taken into account that LVEF is strongly influenced by parameters such as preload and afterload. These factors can distort the interpretation of LVEF and must be carefully considered when using the latter as a diagnostic and prognostic tool in patients with CS.60
At this point, echocardiography is also useful for evaluating segmental contractility disorders, affording crucial information, since these alterations suggest acute coronary syndrome (ACS) as the underlying cause.61
In relation to the right ventricle, and in addition to evaluation of its size with respect to that of the LV, a basic parameter for assessing RV systolic function is tricuspid annular plane systolic excursion (TAPSE). This parameter is measured in the apical four-chamber (4C) view, and calculates the degree of longitudinal displacement of the annular segment of the RV from end-diastole to the systolic peak. A value < 17 mm is suggestive of RV systolic dysfunction.62
Likewise, in this phase, the existence of severe valve disease or cardiac tamponade should be discarded.63
Advanced echocardiographyAdvanced echocardiographic study aims to confirm the etiology, perform a hemodynamic assessment, guide the initial treatment response, and provide orientation as to the need for mechanical circulatory assist devices.
Evaluation of left ventricular functionEchocardiography can provide a noninvasive estimate of systolic volume based on pulsed Doppler evaluation of the left ventricular outflow tract (LVOT),64 and is useful for monitoring the response to treatment.65 Measurement is made as the product of the cross-sectional area of the LVOT and the velocity-time integral (VTI) of aortic flow in the LVOT (Fig. 2). The evaluation of VTI alone has been taken as a surrogate for CO and has been successfully used, especially in quantifying volume response.6 Likewise, echocardiography allows the noninvasive measurement of CP.36
Lastly, tissue Doppler echocardiographic analysis of the systolic wave of the mitral ring or using myocardial strain could constitute a more reliable parameter than LVEF, being able to identify comparatively earlier and smaller myocardial changes and representing a good early prognostic indicator in patients with CS complicating AMI.66
Evaluation of diastolic functionEchocardiography allows the assessment of diastolic function and filling pressures based on the pulsed Doppler study of transmitral flow (E wave) and tissue Doppler study of the mitral ring (eʹ wave) 67,68 (Fig. 2). By considering the E/eʹ ratio, an assessment can be made of the LV filling pressures, where E/eʹ > 14 indicates high pulmonary capillary pressures and E/eʹ < 8 is indicative of normal pressures.69 A recent retrospective study showed that several echocardiographic parameters (including a low systolic volume index and high E/eʹ ratio) are correlated with the SCAI classification of CS and mortality.70
Evaluation of right ventricular functionThe simplified Bernoulli equation allows us to calculate the pressure gradient between the right atrium and ventricle based on continuous Doppler recording of the tricuspid regurgitation trace. On incorporating RAP, this calculation offers an estimate of systolic pulmonary artery pressure (PAPs).
For the study of the systolic function of the right ventricle, and in addition to TAPSE – the main limitations of which are dependence upon loading, a decrease in the context of atrial fibrillation, and cases of regional dysfunction – we can select parameters such as the tissue doppler systolic wave of the tricuspid ring or the fractional area change (Fig. 2).
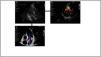
Mechanical complications in ischemic cardiogenic shockSerial echocardiography can detect mechanical complications in cases of AMI, such as left or right ventricle free wall rupture, interventricular communication or complications related to mechanical circulatory support (Fig. 3). In this latter scenario it may even be of great help in controlling insertion of the cannula and in monitoring the recovery of cardiac function, as well as in deciding the adequate timing of weaning.71
Phenotypes of cardiogenic shock, beyond echocardiographyExamination of the SCAI scale shows that within each grade there are different levels of severity according to the phenotype of shock, with three differential prognostic profiles2,72:
- 1
Non-congestive: isolated left-side dysfunction without congestion.
- 2
Cardiorenal: significant left-side dysfunction with kidney damage and lung congestion.
- 3
Cardiometabolic or hemometabolic: right ventricular dysfunction, systemic congestion, hyperlactatemia and multiorgan dysfunction.
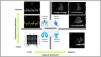
Clinical ultrasound allows us to evaluate the degree of pulmonary as well as systemic congestion, thus adding prognostic and therapeutic value (Fig. 4). Lung ultrasound can identify and quantify lung edema based on counting of the B lines,73 and can be used to monitor patient response to decongesting treatment. On the other hand, the study of flow pulsatility in venous vessels of the abdominal compartment (suprahepatic, portal and renal veins) in the context of the venous excess ultrasound (VExUS) score74 can establish the degree of systemic congestion - though its usefulness in CS has not yet been studied.75
Study of congestion in cardiogenic shock.
A: Pulsed doppler study of transmitral flow. B: Tissue doppler study of the lateral zone of the mitral ring. C: Pulsed doppler study of normal flow in the suprahepatic vein; note that it comprises two anterograde waves: a larger systolic wave (S) and a smaller diastolic wave (D), together with a retrograde wave (atrial systole). As the pressures increase in the right atrium, the magnitude of the S wave decreases until (in cases of severe congestion) the S wave reverts its flow. D: Pulsed doppler study of normal flow in the portal vein. E: Pulsed doppler study of normal flow in the renal artery and vein. F: Lung ultrasound study showing the B line pattern. G: Pleuropulmonary and abdominal ultrasound study showing pleural effusion and perihepatic ascites; the asterisk indicates the diaphragm, with the thoracic cavity at right and the abdominal cavity at left.
The main limitation of both transthoracic and transesophageal echocardiography is that neither constitutes a continuous monitoring tool, and both are moreover operator-dependent. Another limitation of transthoracic echocardiography is that it has a poor window in some patients, while transesophageal echocardiography is an invasive technique, with a risk of tracheal, hypopharyngeal, esophageal or gastric lesions.76
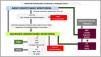
Conclusions. Multimodal monitoring in cardiogenic shockIn sum, the hemodynamic monitoring of CS may be viewed as a multimodal monitoring strategy including and integrating several hemodynamic, metabolic and echocardiographic parameters that offer detailed and complementary information that can describe the characteristics of CS and guide therapeutic interventions (Fig. 5).
Multimodal hemodynamic monitoring in cardiogenic shock.
CP: cardiac power; DO2: oxygen supply; ELWi: extravascular lung water index; GapCO2: venous-arterial pCO2 difference; CO: cardiac output; CFI: cardiac function index; PAPI: pulmonary artery pulsatility index; PVPI: pulmonary vascular permeability index; MBP: mean blood pressure; PAP: pulmonary artery pressure; PAOP: pulmonary artery occlusion pressure; CVP: central venous pressure; ScVO2: central venous oxygen saturation; SvO2: mixed venous oxygen saturation; CRT: capillary refill time; VO2: oxygen consumption; PPV: pulse pressure variation; GEDVi: global end-diastolic volume index.
The authors declare that they have no conflicts of interest.