The ongoing pandemic of COVID-19 declared by the World Health Organization (WHO) due to the new SARS-CoV-2 coronavirus first identified in the city of Wuhan, China in December 2019,1 has put all healthcare systems to the test across the world. The pathophysiology of this infection is still under discussion, and to this date, the effectiveness of the treatments available is still to be confirmed. Hypoxemic respiratory failure is the reason for most admissions in the intensive care unit (ICU), and it mostly requires invasive mechanical ventilation (IMV) in up to 88% of the patients from some of the series reported.2
This is the case of a 53-year-old woman from Nicaragua who used to work at a nursing home. She had a past medical history of hypothyroidism on thyroid hormone replacement treatment, and obesity with a body mass index of 28 kg/m2. She was referred to our ICU from the hospital floor with signs of COVID-19-induced respiratory failure with symptoms of 15-day evolution. She had previously been treated with azithromycin, lopinavir/ritonavir, hydroxychloroquine, and methylprednisolone at doses of 1 mg/kg/24 h. She was treated with high-flow oxygen therapy immediately after admission with torpid evolution that required orotracheal intubation and IMV after 48 h due to respiratory claudication. It was then that she started treatment with remdesivir for 10 days. It was necessary to implement measures such as neuromuscular relaxation with perfusion of cisatracurium, ventilation in the prone position, and inhaled nitric oxide (iNO). Despite all this, it was impossible to proceed with protective ventilation (plateau pressure >30 mbar, and a driving pressure of 20 mbar). The patient remained on refractory hypoxemia (PaO2/FiO2 < 100) and respiratory acidosis. Given the circumstances and after 5 days on IMV, it was decided to initiate treatment with veno-venous extracorporeal membrane oxygenation (VV-ECMO) via femorojugular cannulation.
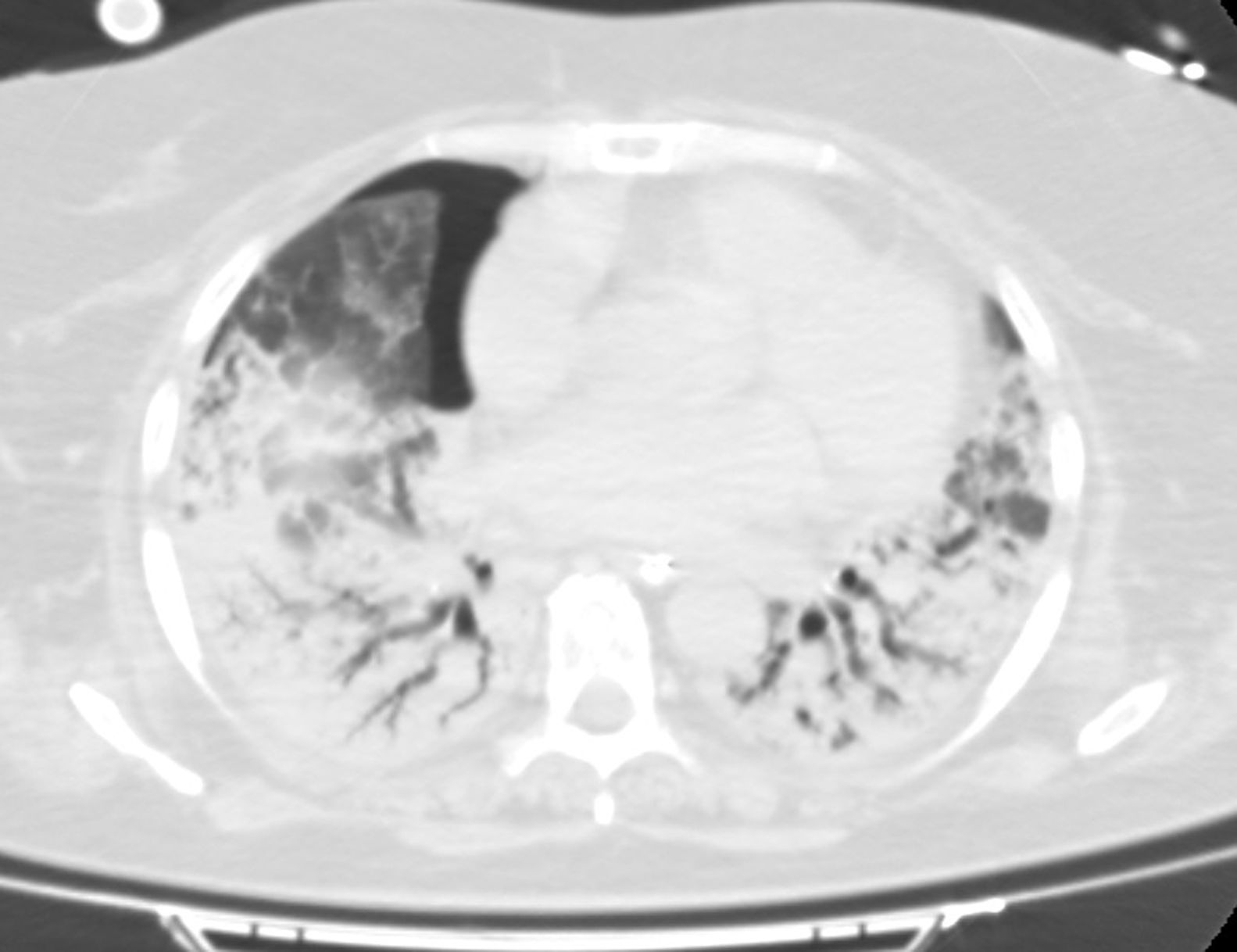
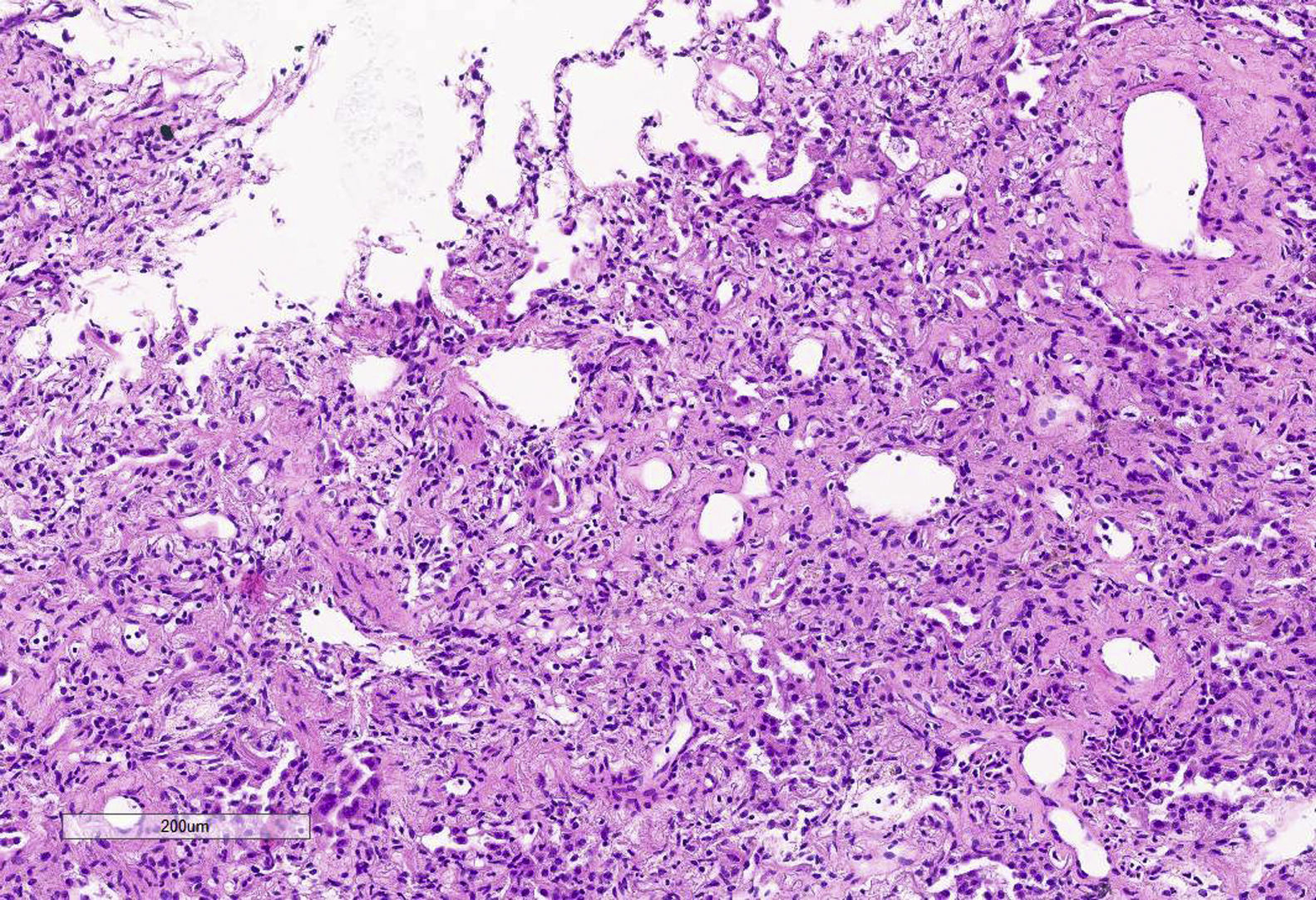
After ECMO implementation, PaO2 and PaCO2 came back to normal ranges. Similarly, ultraprotective ventilation was attempted with the following parameters: tidal volume (TV) of 3 mL/kg (210 mL), respiratory rate of 10 rpm, a PEEP of 10cmH2O, and a FiO2 of 30%. With it, plateau pressure reached >30 mbar with a dynamic compliance of 5 mL/mbar. In an attempt to reach a more protective ventilation the TV was reduced down to 130 mL. After 20 days on this ventilatory pattern of poor prognosis it was suggested to acquire CT images of the thorax and perform a pulmonary cryobiopsy. The CT scan (Fig. 1) revealed the presence of an extensive air bronchogram with small anterior regions of ground-glass opacities but no clear signs of pulmonary fibrosis. We should mention the incidental finding of a small anterior pneumothorax cavity not seen on the x-rays or ultrasounds before that, once drained, was not followed by changes in the pulmonary dynamics. The pulmonary biopsy (Fig. 2) confirmed a pattern of diffuse alveolar damage (DAD) in the proliferative phase and progression to fibrosis.
After these findings and given the patient’s situation of respiratory irreversibility, the family was informed and they later gave their informed consent to adequate the therapeutic effort and withdraw ECMO support after 28 days on this therapy, eventually leading to the patient’s immediate death.
Acute respiratory distress syndrome (ARDS) is characterized by the presence of non-cardiogenic pulmonary edema that triggers the appearance of hypoxemia and bilateral infiltrates on the thoracic x-ray. Several diseases and conditions can progress into a respiratory situation like this including viral pneumonia as we recently saw in numerous patients with SARS-CoV-2-induced infections that required IMV.3
The possible progression of advanced acute respiratory distress into a condition of irreversible pulmonary fibrosis is well-known.4 Assessments through imaging modalities like the CT scan is important because of their prognostic utility regarding the presence of established fibrosis.5 Several studies have associated certain findings in the imaging modalities with the final stages of ARDS and fibrosis6: “traction bronchiectasis, ground-glass opacities, damage to >80% of the pulmonary parenchyma, honeycomb lung or signs of high pressures in the right ventricle (RV) (RV dilatation, and a larger pulmonary artery diameter)”. On the contrary, the presence of a consolidation pattern in its early stages is associated with a more favorable disease progression.7 Also, the association between low pulmonary compliance and the presence of reticulations and bronchiectasis on the CT images has been reported.8
Given our patient’s uncertain prognosis, and the restrictive patterns seen on the ventilator console it was decided to perform a thoracic CT scan to confirm the suspected poor prognosis. However, the radiological images obtained were not consistent with restrictive pulmonary dynamics or irreversible pulmonary fibrosis. The finding of an extensive consolidation pattern may have limited the assessment of fibrosis9 which, on the other hand, contrasts with the more favorable disease progression reported in previous reviews.7 Still, we should mention that findings of pneumothorax in late distress stages have been associated with a higher mortality rate.10
Therefore, given the totally unknown stage of the disease it was decided to obtain a sample from the pulmonary tissue. The tissue sample obtained showed a significant hyperplasia of pneumocytes, and prominent thickening of the alveolar septa at the expense of hyaline fibrosis with obliteration of alveolar airspaces. The inflammatory component was very scarce, and no hyaline membranes or thrombi could be identified.
We found a significant histo-radiological dissociation where the in vivo microscopic visualization of a tissue sample was key in the decision-making process. What was new about this case was the histological images obtained in a respiratory situation of total incompatibility with life (compliance of 5 mL/mbar) where the in vivo tissue sample was obtained thanks to ECMO support.
We brought up this case here, as well as the data collected to add evidence on this new and unknown entity that is brand new to our medical specialty.
Please cite this article as: Peiro Chamarro M, Ruiz de Gopegui Miguelena P, Sampedro Martín I, Callau Calvo A, Martínez Lamazares MT, Fuertes Schott C. Disociación histo-radiológica en fibrosis pulmonar secundaria a infección por SARS-CoV-2. Med Intensiva. 2021;45:e34–e36.