To describe and evaluate the impact of a system for early detection and intervention in patients at risk outside the ICU upon the outcome of patients admitted to the ICU and the number of cases of hospital cardiopulmonary arrest.
SettingA second-level hospital in the Community of Madrid (Spain) with electronic clinical histories.
MethodsAn intensivist reviewed each of the patients meeting the inclusion criteria, and decided the need or not for intervention. Posteriorly, in collaboration with the physician supervising the patient, the needed level of care was decided, along with the subsequent management protocol.
DesignA descriptive and quasi-experimental “before-after” study was made.
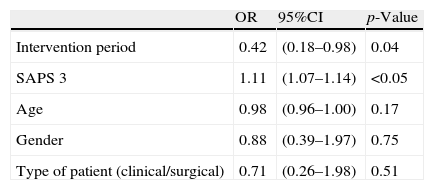
ResultsA total of 202 patients were intervened during the study period, with the inclusion of 147 patients after detecting altered laboratory test results through our software application. During the control period, the mortality rate in the ICU was 9%, versus 4.4% during the intervention period (p=0.03). In the multivariate analysis, the two factors significantly related to mortality were admission during the intervention period (OR=0.42; 95%CI: 0.18–0.98; p=0.04) and SAPS 3 (OR=1.11; 95%CI: 1.07–1.14; p<0.05). There were 10 cardiopulmonary arrest alerts during the control period, versus three in the intervention period (p=0.07).
ConclusionsEarly detection activities in patients at risk outside the ICU can have beneficial effects upon the patients admitted to the ICU, and can contribute to reduce the number of hospital cardiopulmonary arrests.
Describir y evaluar la repercusión de un sistema de detección e intervención precoz en pacientes de riesgo fuera de la UCI en la evolución de los pacientes ingresados en UCI y el número de paradas cardiorrespiratorias (PCR) hospitalarias.
ÁmbitoHospital de nivel 2 en la Comunidad de Madrid con historia clínica electrónica.
MétodosUn intensivista revisa cada uno de los pacientes que cumplan los criterios de inclusión y decide la necesidad o no de intervención. Posteriormente, junto al médico a cargo del paciente, se determina cuál es el nivel de cuidados que necesita y se decide la pauta a seguir a continuación.
DiseñoEstudio descriptivo y cuasi-experimental «before-after».
ResultadosEn el periodo de estudio se intervino en un total de 202 pacientes. Ciento cuarenta y siete fueron incluidos tras detectarse analíticas alteradas a través de nuestro programa informático. En el periodo de control la mortalidad en UCI fue 9 frente al 4,4% en el periodo de intervención (p=0,03). En el análisis multivariable, los 2 factores que guardaron relación significativa con la mortalidad fueron el haber ingresado durante el periodo de intervención OR 0,42 (IC95%; 0,18 a 0,98) (p=0,04) y el SAPS 3 OR 1,11 (IC95%; 1,07 a 1,14) (p<0,05). El número de avisos por PCR en el periodo control fue 10 frente 3 en el periodo de intervención (p=0,07).
ConclusionesLa actividad de detección precoz de pacientes en riesgo fuera de la UCI puede producir un efecto beneficioso sobre los pacientes ingresados en UCI así como una reducción de las PCR hospitalarias.
The aim of Intensive Care Medicine and of the Intensive Care Unit (ICU) is to offer critically ill patients quality treatment adjusted to their needs, and in the safest manner possible.1 In the United States, it is considered that over one-half of the population will require admission to the ICU at some point in life, and that an important percentage of individuals will die in such Units, consuming between 0.5 and 1% of the gross domestic product.2
A broad and more balanced hospital perspective is needed for the treatment of patients at risk, classifying them according to the required level of care and not according to where they happen to be located. Hospitalization of the seriously ill patient should be regarded as continuous from before to after admission to the ICU.3,4
Delays in the treatment of hospitalized patients often result in emergency admission to the ICU, which in turn implies a prolongation of hospital stay and even increased mortality.5 It has been estimated that over 50% of hospitalized patients failed to receive optimum management before admission to the ICU, and that 40% of all admissions to the Unit are in fact avoidable.6 On the other hand, delays in admission to the ICU – mainly due to a limitation or shortage of available beds – has been associated to mortality, as described by Cardoso et al.,7 who reported a 1.5% and 1% mortality increase in the ICU and in hospital, respectively, for every hour of delayed admission. On considering those patients who after discharge from the ICU require subsequent readmission to the Unit because of clinical worsening, mortality in the ICU and the duration of hospital stay increase 4- and 2.5-fold, respectively.8
It must be taken into account that life-threatening situations are usually preceded by physiopathological alterations that are detectable and avoidable. This is particularly relevant in the case of the so-called “time-dependent” diseases such as sepsis, acute coronary syndrome and cardiac arrest.9–12
On the basis of these premises, we raised the hypothesis that management of the seriously ill patient, while centered on the ICU, can be extended beyond the Unit, constituting a continuous process throughout the duration of patient hospital stay. Any change within the process can have a positive impact upon patient outcome. The aim of this study was to describe a system designed to allow early detection and intervention in patients at risk outside the ICU and to evaluate its repercussions upon the course of those patients admitted to Intensive Care and upon the number of cases of in-hospital cardiac arrest.
Patients and methodsStudy settingThe study was carried out in a 210-bed second level hospital in the Community of Madrid (Spain), with a polyvalent adult clinical–surgical ICU (8 beds). The hospital is fully digitalized with a common electronic clinical record (Selene®) and data exploitation software (Datawarehouse®).
MethodsDuring the intervention period, on working days, one of the intensivists reviews each of the patients meeting the inclusion criteria based on the electronic clinical record in Selene®, and decides whether intervention is needed or not. Posteriorly, together with the physician in charge of the patient, the required level of care is decided, and the subsequent actions are defined.
From our information system, linked to the laboratory test managing program, a daily download is produced of all the laboratory test data requested in the hospital during the previous 24h. This database is in turn processed by customized software designed to identify all those test parameter values that exceed certain predefined limits. In this way we obtain a form with the altered parameter, the time of extraction and its value, and relate the information to the patient and his or her location. The selected laboratory test parameters are troponin I>0.3μg/l, pH<7.30, pCO2>60mmHg, platelets<100,000/μl, and lactate>3mmol/l. These values were predefined by the investigators in order to identify patients with incipient organ damage, fundamentally in the context of sepsis, respiratory failure and myocardial damage.
PatientsInclusion criteria- 1.
Patients with laboratory test alterations detected by means of the software.
- 2.
Patients at risk of a poor clinical course in accordance with the criterion of other specialists.
- 3.
Patients evaluated the previous day by the intensivist on duty, and who are regarded by the latter as requiring follow-up but without the need for admission to the ICU at that time.
- 4.
Patients discharged from the ICU and who meet some of the following criteria and are regarded by the intensivist supervising discharge as requiring follow-up: laboratory test alteration, prolonged stay in the ICU, tracheostomy (performed in the ICU), noninvasive mechanical ventilation (started in the ICU), comorbidity or request on the part of the receiving physician.
- 1.
Patients with explicit life support limitation (LSL) instructions.
- 2.
Patients considered to have terminal disease, subjected to palliative care.
- 3.
Pediatric patients.
The interventions among the included patients comprise the following:
- 1.
Confirmation of patient stability and good evolution, without the need for further actions.
- 2.
Participation in LSL decision.
- 3.
Diagnostic reorientation.
- 4.
Intensification or adjustment of therapeutic measures.
- 5.
Close follow-up over the subsequent hours and clinical evaluation in the course of duty on the day of detection.
- 6.
Early admission to the ICU or readmission to the unit.
The study consisted of two parts:
- 1.
A descriptive study covering the period between July 2011 and January 2012, when the aforementioned technological solution was adopted, allowing the analysis of laboratory test data throughout the hospital, with collaboration among the different specialties and duty follow-up.
- 2.
A quasi-experimental “before-after” study comparing the periods before and after introduction of the software system. The corresponding control period was the above-mentioned preceding interval between July 2011 and January 2012.
The statistical analysis was carried out using the G-STAT 2.0.1® package. The normal or non-normal distribution of quantitative variables was evaluated using the Kolmogorov–Smirnov test. These variables are reported as the mean and range as dispersion measure, or as the mean and standard deviation. Qualitative variables in turn are expressed as frequencies and percentages.
The comparison of qualitative variables was carried out with the chi-squared test. The comparison of quantitative variables in turn was carried out using the Student's t-test in the presence of a normal distribution, or the Mann–Whitney U-test in the case of a non-normal distribution. The data are reported as the mean and standard deviation.
Risk association was assessed using multivariate analysis, and introducing all the studied variables which could be clinically related to survival. Statistical significance was considered for p<0.05 in all cases.
Since the patients presented different characteristics, improved understanding of the data was ensured by establishing two groups: a first “out-ICU activity” group including the patients detected by the software system, those identified by the intensivist on duty, and those commented by the rest of the specialists; and a second “post-ICU follow-up” group comprising the patients subjected to follow-up after discharge from the ICU. The study was approved by the research ethics committee.
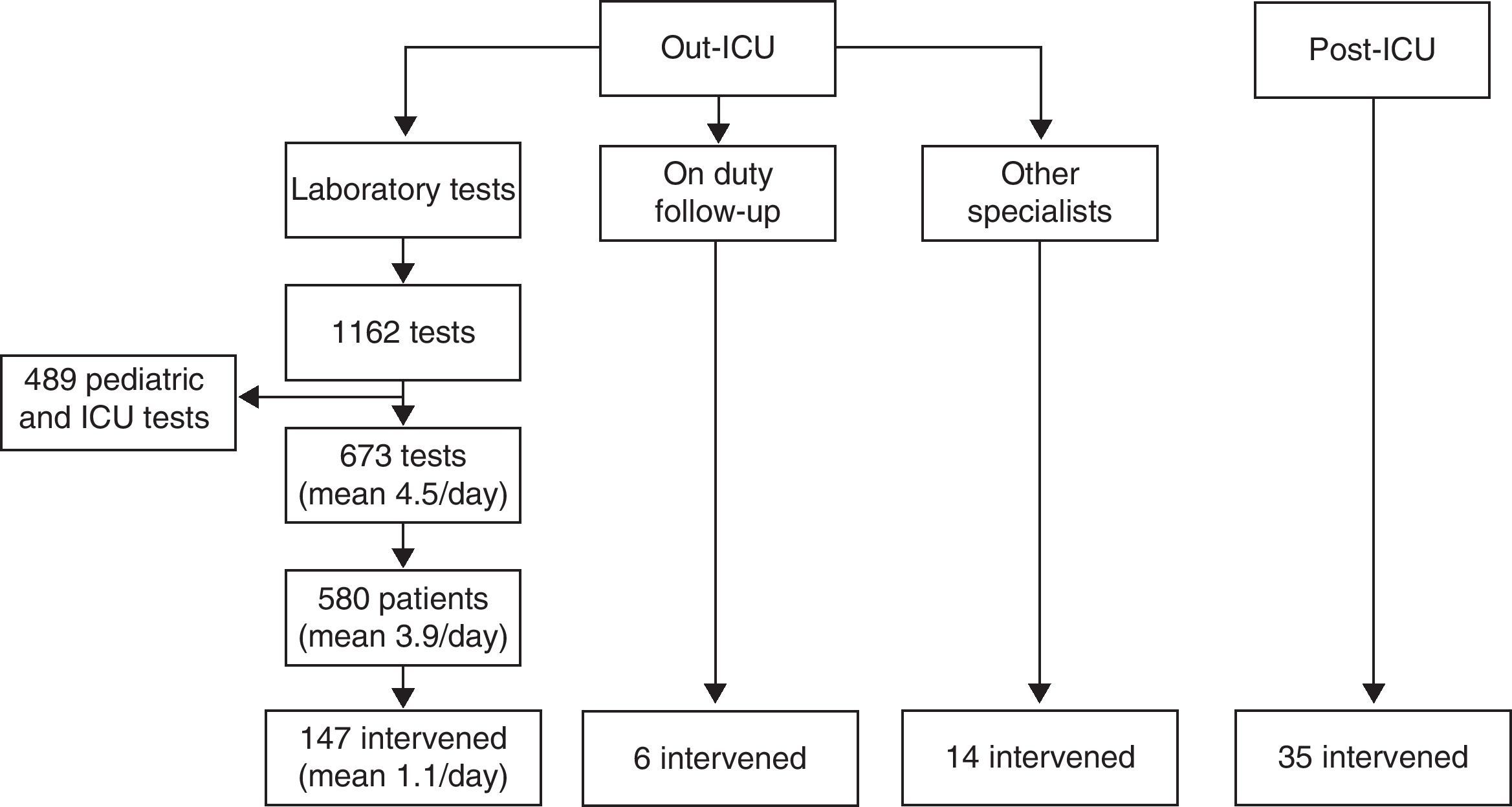
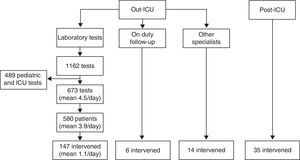
ResultsThe hospitalized patients included in the intervention period are described in Fig. 1. Tables 1 and 2 respectively show the demographic variables of the patients in the “out-ICU activity” and “post-ICU follow-up” groups.
Patients intervened. Of the patients in the “out-ICU activity” group detected by the software system, and after discarding from the 1162 laboratory tests those corresponding to pediatrics and the ICU, we reviewed the clinical records of 580 patients, with final intervention in 147 cases. To these we added 6 patients detected by the intensivist on duty the day before, and 14 cases commented by the other specialists. In the “post-ICU follow-up” group, follow-up was made of 35 patients considered to be at risk of presenting a poor clinical course.
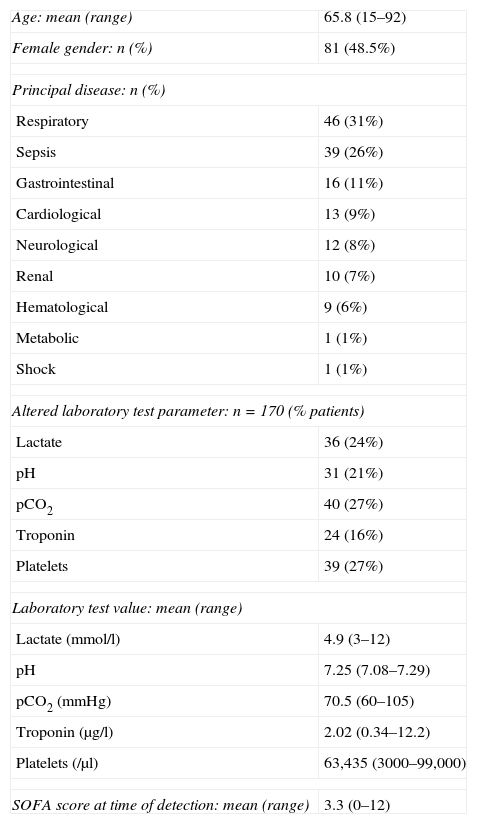
“Out-ICU activity” demographic data.
| Age: mean (range) | 65.8 (15–92) |
| Female gender: n (%) | 81 (48.5%) |
| Principal disease: n (%) | |
| Respiratory | 46 (31%) |
| Sepsis | 39 (26%) |
| Gastrointestinal | 16 (11%) |
| Cardiological | 13 (9%) |
| Neurological | 12 (8%) |
| Renal | 10 (7%) |
| Hematological | 9 (6%) |
| Metabolic | 1 (1%) |
| Shock | 1 (1%) |
| Altered laboratory test parameter: n=170 (% patients) | |
| Lactate | 36 (24%) |
| pH | 31 (21%) |
| pCO2 | 40 (27%) |
| Troponin | 24 (16%) |
| Platelets | 39 (27%) |
| Laboratory test value: mean (range) | |
| Lactate (mmol/l) | 4.9 (3–12) |
| pH | 7.25 (7.08–7.29) |
| pCO2 (mmHg) | 70.5 (60–105) |
| Troponin (μg/l) | 2.02 (0.34–12.2) |
| Platelets (/μl) | 63,435 (3000–99,000) |
| SOFA score at time of detection: mean (range) | 3.3 (0–12) |
SOFA, Sequential Organ Failure Assessment.
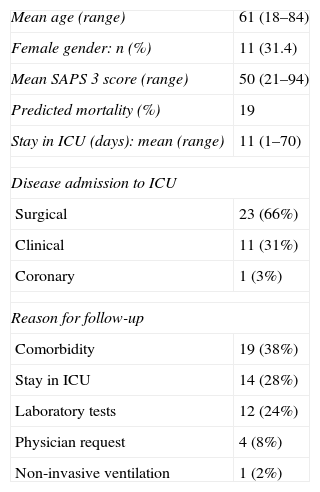
“Post-ICU follow-up” demographic data.
| Mean age (range) | 61 (18–84) |
| Female gender: n (%) | 11 (31.4) |
| Mean SAPS 3 score (range) | 50 (21–94) |
| Predicted mortality (%) | 19 |
| Stay in ICU (days): mean (range) | 11 (1–70) |
| Disease admission to ICU | |
| Surgical | 23 (66%) |
| Clinical | 11 (31%) |
| Coronary | 1 (3%) |
| Reason for follow-up | |
| Comorbidity | 19 (38%) |
| Stay in ICU | 14 (28%) |
| Laboratory tests | 12 (24%) |
| Physician request | 4 (8%) |
| Non-invasive ventilation | 1 (2%) |
SAPS 3, Simplified Acute Physiology Score; ICU, Intensive Care Unit.
Of the detected patients in the “out-ICU activity” group, the majority (n=108; 65%) came from the Emergency Care area, followed by the clinical ward (n=55; 33%) and the surgery and resuscitation ward (n=4; 3%). Of the total interventions (n=233) in this group, 45% consisted of medical assessment without any further actions, thereby confirming the stability of the patient; 18% comprised participation in consensus-based LSL decisions; 12% consisted of close patient monitoring over the subsequent hours in the course of duty on the day of detection; 14% involved diagnostic reorientation with the physician in charge of the patient; 8% comprised intensification or adjustment of the therapeutic measures; and 3% (6 patients) involved early admission to the ICU.
Of these intervened patients, 5 (3%) had been admitted to the ICU within the same process, and only four (2.4%) involved calling of the specialist on duty after the end of follow-up. The mean duration of follow-up was one day (range 1–6), with a mortality rate of 7.2%. In relation to the 12 patients who died, LSL had been decided previously between the family and the physician in 7 cases.
Regarding the patients of the “post-ICU follow-up” group, with a total of 104 interventions, the most frequent action was medical assessment (46% of the cases), followed by diagnostic reorientation (26%), treatment adjustment (13%), and follow-up in the course of duty (12%). One patient was readmitted to the ICU during follow-up, LSL was agreed with the supervising physician in another case, and one patient was moved to another hospital because the required specialized care was not available in our center. The mean duration of follow-up was 3 days (range 1–11); the mean hospital stay after discharge from the ICU was 13 days (range 2–34); in-hospital mortality was 0%; and only one patient involved calling of the specialist on duty, with admission after the end of follow-up.
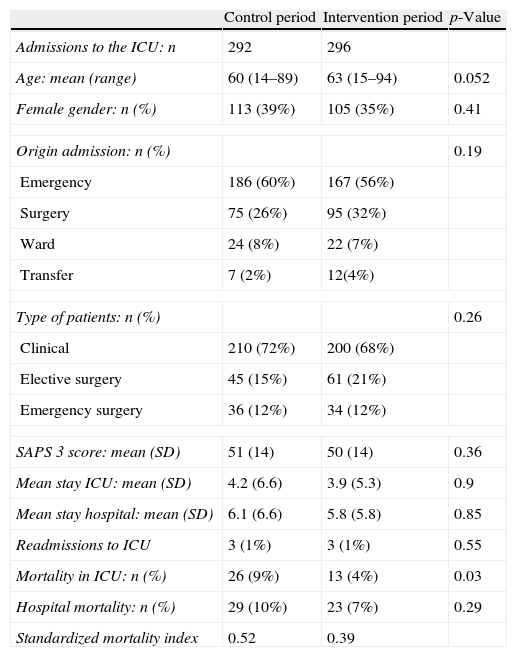
The characteristics of the patients admitted to the ICU in the control period versus the intervention period are shown in Table 3. The mortality rate predicted by the SAPS 3 (Simplified Acute Physiology Score) in both groups was 19%, while actual mortality in the ICU was 9% in the control period and 4% in the intervention period (p=0.03). In the multivariate analysis (Table 4), with in-ICU mortality as the dependent variable, the two factors found to be significantly related to the mentioned variable were admission during the intervention period (OR 0.42 [95%CI: 0.18–0.98]) (p=0.04) and the SAPS 3 score (OR 1.11 [95%CI: 1.07–1.14]) (p<0.05).
Characteristics and evolution of the patients admitted to the ICU.
| Control period | Intervention period | p-Value | |
| Admissions to the ICU: n | 292 | 296 | |
| Age: mean (range) | 60 (14–89) | 63 (15–94) | 0.052 |
| Female gender: n (%) | 113 (39%) | 105 (35%) | 0.41 |
| Origin admission: n (%) | 0.19 | ||
| Emergency | 186 (60%) | 167 (56%) | |
| Surgery | 75 (26%) | 95 (32%) | |
| Ward | 24 (8%) | 22 (7%) | |
| Transfer | 7 (2%) | 12(4%) | |
| Type of patients: n (%) | 0.26 | ||
| Clinical | 210 (72%) | 200 (68%) | |
| Elective surgery | 45 (15%) | 61 (21%) | |
| Emergency surgery | 36 (12%) | 34 (12%) | |
| SAPS 3 score: mean (SD) | 51 (14) | 50 (14) | 0.36 |
| Mean stay ICU: mean (SD) | 4.2 (6.6) | 3.9 (5.3) | 0.9 |
| Mean stay hospital: mean (SD) | 6.1 (6.6) | 5.8 (5.8) | 0.85 |
| Readmissions to ICU | 3 (1%) | 3 (1%) | 0.55 |
| Mortality in ICU: n (%) | 26 (9%) | 13 (4%) | 0.03 |
| Hospital mortality: n (%) | 29 (10%) | 23 (7%) | 0.29 |
| Standardized mortality index | 0.52 | 0.39 | |
SD, standard deviation; SAPS, Simplified Acute Physiology Score.
Standardized mortality index: actual mortality/mortality predicted by SAPS 3.
The number of alerts due to in-hospital cardiac arrest in the control period was 10, versus three in the intervention period (p=0.07).
In the intervention period we recorded a significant reduction in consultation calls (non-emergencies) to the intensivist on duty, compared with the control period (385 [70%] vs 361 [64%]; p=0.02), with a significant increase in calls during the morning shift (220 [40%] vs 279 [49%]; p=0.005) and in the number of admissions to the ICU during the morning shift (96 [33%] vs 125 [42%]; p=0.01).
DiscussionIn the present study, “out-ICU activity” comprised direct intervention by the Department of Intensive Care Medicine in 167 patients – 97% of which were located in the Emergency Care area and conventional clinical wards. Only 6 of these patients (4%) required early admission to the ICU, with an in-hospital survival rate of 93%. On the other hand, “post-ICU follow-up” comprised intervention in 35 patients, and fundamentally affected surgical patients (66%). Of these patients, only one required readmission to the ICU after the end of follow-up, with an in-hospital survival rate of 100%.
Regarding the quasi-experimental “before-after” study, the patients admitted to the ICU (with similar degrees of severity at the time of admission) showed a significant decrease in mortality–with far lower values in both study periods than predicted by the severity scores at the time of admission to the ICU. During the two mentioned periods there were no significant changes in patient care or in the protocols capable of explaining a change in mortality rate. This circumstance may represent a multifactorial effect related to better selection of the patients amenable to admission to the ICU, associated to an increased possibility of admission of elective surgical patients and an earlier admission of patients at risk. In order to confirm an association, however, we of course would need a larger sample size, and perhaps the conduction of a multicenter trial. In the case of in-hospital cardiac arrest, the observed decrease was clearly related to the mentioned activity.
The aim of this project is to modify the work flow in the ICU, with improved programming of admissions, the reduction of cardiac arrest in the hospital, and improvements in efficiency and prognosis among the patients admitted to the ICU–thereby contributing to improve healthcare resource management. Such benefits are probably attributable to a decrease in emergency admissions due to delays in treatment and in delays in admission to the Unit because of a shortage of beds, which have been found to be associated to increased mortality and hospital stay.5–8,13
We based the project design on the fact that life-threatening situations are usually preceded by detectable and preventable physiopathological alterations–this being particularly relevant in the case of the so-called “time-dependent” diseases such as sepsis, acute coronary syndrome and cardiac arrest.9–12 In this context, hospital rapid response teams have already been established with the same purpose of identifying patients at risk outside the ICU.14,15 In the United States, these teams form part of the “five million lives” campaign of the Institute for Healthcare Improvement, aimed at improving patient prognosis and reducing the number of deaths.16 “Code” type intervention systems have also been developed for clinical conditions such as sepsis, under the guidelines of the Surviving Sepsis Campaign,17,18 infarction19 or stroke,20 and have demonstrated their effectiveness. To give us an idea of the impact of such measures upon resource utilization, we can consider the case of severe sepsis. Its annual incidence is 14.1 cases/10,000 inhabitants, with an overall mortality rate of 33% (with higher percentages in the case of more than one organ dysfunction, liver dysfunction or the presence of comorbidities). The mean hospital stay among such patients in our setting is 28.9 days, and the annual cost of severe sepsis care in the Community of Madrid alone totals 70 million euros.21
For the implementation of this project we made use of information technology, which improves patient care, as it has been shown by other studies (including the RIFLE study).22–26 The software used allows the detection of altered laboratory test values–thereby expanding early detection and intervention to the entire hospital.
The literature offers contradictory data on the usefulness of different systems for the early identification of patients at risk. Nevertheless, experience has been gained based on the creation of rapid response teams, involving different activation systems, which have been shown to offer benefit. In this sense, a recent systematic review of the literature has reported a 34% reduction in hospital cardiac arrest outside the ICU.27 The rapid response teams are evolving, with changes in the activation systems used, and involving the inclusion of laboratory test data.28
As limitations to our study, mention must be made of the fact that during the control period we in some way carried out activities outside the ICU, with daily reviews of the patients exclusively located in the Emergency Care observation area and the implementation of “code” type intervention systems such as the Sepsis Code, Acute Coronary Syndrome Code and Cardiopulmonary Resuscitation Code. However, such bias favors the control group, not the intervention group. On the other hand, the decision to intervene in the case of a patient meeting the inclusion criteria was left to the intensivist in charge of the activity that day–a situation that adds subjectiveness in relation to the selection of patients for intervention. Lastly, it must be taken into account that this was a non-randomized, single center study with a limited sample size.
ConclusionsThe early detection of patients at risk outside the ICU can offer benefit in relation to the patients admitted to the ICU, and can result in fewer in-hospital cardiac arrests.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Abella Álvarez A, et al. Proyecto UCI sin paredes. Efecto de la detección precoz de los pacientes de riesgo. Med Intensiva. 2013;37:12–8.