Important recent insights have emerged regarding the cellular and molecular role of carbon dioxide (CO2) and the effects of hypercapnia. The latter may have beneficial effects in patients with acute lung injury, affording reductions in pulmonary inflammation, lessened oxidative alveolar damage, and the regulation of innate immunity and host defenses by inhibiting the expression of inflammatory cytokines. However, other studies suggest that CO2 can have deleterious effects upon the lung, reducing alveolar wound repair in lung injury, decreasing the rate of reabsorption of alveolar fluid, and inhibiting alveolar cell proliferation. Clearly, hypercapnia has both beneficial and harmful consequences, and it is important to determine the net effect under specific conditions. The purpose of this review is to describe the immunological and physiological effects of carbon dioxide, considering their potential consequences in patients with acute respiratory failure.
En los últimos años han surgido importantes descubrimientos sobre el papel del dióxido de carbono (CO2) a nivel celular y molecular, y sobre los efectos de la hipercapnia. Esta última puede tener efectos beneficiosos en pacientes con patología pulmonar aguda, como la reducción de la inflamación pulmonar y del daño oxidativo alveolar, la regulación de la inmunidad innata, la defensa del huésped y la inhibición de la expresión de citoquinas inflamatorias. Sin embargo, otros estudios sugieren que el CO2 puede tener efectos nocivos en el pulmón, como retraso en la reparación alveolar tras la injuria pulmonar, disminución de las tasas de reabsorción del fluido alveolar e inhibición de la proliferación de células alveolares. Por lo tanto, la hipercapnia tiene efectos tanto beneficiosos como nocivos y es importante determinar el efecto neto en condiciones específicas. El propósito de esta revisión es describir los efectos fisiológicos e inmunomoduladores de la hipercapnia, considerando sus potenciales consecuencias en el paciente con insuficiencia respiratoria aguda.
In the critical patient with acute respiratory failure subjected to protective ventilation with low tidal volumes (Vt),1–3 elevation of the carbon dioxide (CO2) levels is allowed in order to avoid ventilator-induced lung injury (VILI). In the past, permissive hypercapnia (PH) was accepted because there were no options for the treatment of respiratory acidosis other than the use of a corrective buffer.
However, in recent years decapneization techniques involving extracorporeal CO2 removal (ECCO2R) have been introduced with the purpose of further reducing Vt while also avoiding VILI and hypercapnia.
It is in this context where CO2 regains importance, since we now have techniques that can reduce its levels. However, should we really prevent or correct hypercapnia in patients with severe acute respiratory failure? In recent years studies have been made in an attempt to clarify the impact of CO2 as a biological agent with effects at cellular and systemic level – with controversial results.
The present article reviews the effects of CO2 and its actions at physiological and biological level, as well as its role within the clinical context of the critically ill patient, focusing on acute respiratory distress syndrome (ARDS), with the purpose of answering the above question.
Physiological effectsCarbon dioxide produces a number of different physiological effects in the body (see Table 1e of supplementary material).
Respiratory effectsAt respiratory level, CO2 plays an important role in relation to both oxygenation and lung mechanics.
In experimental models, moderate hypercapnia (FiCO2 5% [PaCO2=50–60mmHg]) improves arterial oxygenation in both healthy and diseased lungs4–6 by reducing ventilation/perfusion (V/Q) heterogeneity. However, at clinical level it has been seen that hypercapnia in patients with ARDS subjected to protective ventilation produces hypoxemia secondary to an increase in the pulmonary short-circuit (intrapulmonary shunt), generating West zones 3 (V/Q<1) resulting from the combination of increased pulmonary flow and alveolar hypoventilation.7
With regard to lung mechanics, hypercapnia has been seen to produce an increase in lung distensibility through modulation of the interaction between actin and myosin at pulmonary parenchymal level,8 and possibly through an increase in production and improvement of the properties of surfactant.9
With regard to diaphragmatic function, the role played by hypercapnia is subject to some controversy. Experimental studies have found hypercapnia to preserve the contractility of the diaphragm and prevent its dysfunction, probably due to a decrease in both inflammatory response and myosin loss at diaphragmatic level.10,11 However, at clinical level, hypercapnia has been shown to produce diaphragmatic dysfunction in patients under conditions of spontaneous ventilation, as a result of alterations in electrical signal transmission of the afferent pathway of the phrenic nerve.12 The clinical impact of hypercapnia upon diaphragmatic function remains to be defined, particularly in patients in which weaning and release from mechanical ventilation (MV) is sought.
The CO2 levels appear to play a role in relation to airway resistance through the modulation of smooth muscle tone. However, CO2 may increase, decrease or have no effect upon lung resistances. This variability may be due to the site where CO2 exerts its effect. In effect, it has been seen that hypercapnia at local alveolar level relaxes the small bronchi, secondary to modulation of Ca2+ influx to the bronchial smooth muscle cells.13 However, hypercapnia at systemic level produces bronchoconstriction mediated by vagus nerve stimulation.14
Hemodynamic effectsAt cardiovascular level, hypercapnic acidosis produces a net stimulating effect through activation of the sympathetic – adrenergic axis, with an increase in cardiac output secondary to a rise in preload and heart rate, and a decrease in afterload. On the other hand, hypercapnia also produces depressor effects at cardiovascular level, with direct inhibition of myocardial15 and smooth muscle cell contractility.16 These effects are independent of the pH levels. Nevertheless, the stimulating effects predominate over the mentioned depressor effects, resulting in an increase in oxygen transport.
Other possible mechanisms underlying the increase in oxygenation could be an increase in oxygen unloading at circulatory level (Bohr effect), or a secondary rise in hematocrit.17
Although the effects of CO2 at cardiovascular level appear to be beneficial, at pulmonary level hypercapnia causes capillary vasoconstriction and increases the mean pulmonary artery pressure. This and the effects of ventilation with positive pressure lead to an increase in right ventricular afterload. The pulmonary artery pressure increase induced by hypercapnia may contribute to the appearance of acute cor pulmonale in patients with ARDS, where a degree of pulmonary hypertension is present - with a resulting increase in mortality.18,19
In turn, pulmonary hypertension could increase capillary wall stress. As a result, in patients with ARDS subjected to mechanical ventilation, the worsening of such stress secondary to hypercapnia could theoretically worsen the lung injury induced by mechanical overdistension.20,21
Cerebrovascular regulationCarbon dioxide is a potent regulator of cerebrovascular tone. Each mmHg change in PaCO2 is associated with a 1–2ml/100g/min change in cerebral blood flow.22
Hypercapnic acidosis produces dilatation of the precapillary arterioles of the brain, with an increase in cerebral blood flow. This is particularly important in patients with diminished cerebral distensibility, where the increase in cerebral blood flow may cause intracranial hypertension.
The probable mechanism whereby CO2 produces such vasodilatation involves activation of the neuronal isoform of nitric oxide synthase (nNOS), increasing the production of nitric oxide (NO), which in turn activates the K+-ATP and K+-Ca channels through the mediation of cGMP, producing a decrease in intracellular calcium with secondary vasodilatation.22
Carbon dioxide is a potent regulator of ventilation through the chemoreceptors located in the ventral portion of the spinal bulb. This is particularly important in critical patients with acute respiratory failure (as in ARDS), where respiratory effort increases the production of CO2 by up to 30%,23 associated to the increase in alveolar dead space (VDALV).24 Such compensating hyperventilation gives rise to a vicious circle, with an increase in respiratory muscle work demand and oxygen consumption, tachypnea, fatigue and claudication. In this scenario, invasive mechanical ventilation proves necessary as a supportive measure – with the deleterious effects associated with its use (e.g., VILI, diaphragmatic dysfunction associated to mechanical ventilation), as well as the effects derived from patient sedation, relaxation and prolonged immobilization.
Biological effectsVentilator-induced lung injury (VILI)Hypercapnia has potential beneficial effects as evidenced by experimental studies in acute lung injury (ALI), such as a decrease in the level of inflammatory mediators or in alveolar oxidative damage. However, a number of studies also suggest that CO2 could have deleterious effects upon the lungs, independently of the pH levels (Table 2e of supplementary material describes the preclinical studies on hypercapnic acidosis, while Table 3e of supplementary material summarizes the immune modulating effects of hypercapnia). The effects of CO2 at pulmonary level are commented below.
Positive effectsDifferent studies have shown hypercapnia to reduce VILI, presumably as a result of a decrease in the damage caused by mechanical overdistension.
Alveolar mechanical overdistension produces deformation of the alveolar structure. The rise in tension and/or disruption of the cytoskeleton and cellular matrix in turn activates specific mechanoreceptors that send signals to the cell, resulting in the release of inflammatory mediators. This mechanism, added to the tissue damage and increase in permeability, can worsen the already existing respiratory distress.25,26
The first study to demonstrate the protective effects of hypercapnic acidosis in a model of VILI was carried out by Broccard et al.27 In their experimental model, isolated rabbit hearts were ventilated with low peak inspiratory pressure (PIP) (15cmH2O) versus high PIP (20–25–30cmH2O) and exposed to hypercapnia or normocapnia. The authors showed hypercapnic acidosis to decrease microvascular permeability, the formation of lung edema, and the protein content in bronchoalveolar lavage (BAL) in the high PIP group.
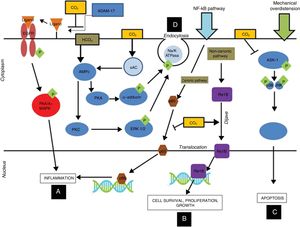
More recent studies found hypercapnia with different concentrations of CO2 (FiCO2 4% [PaCO2=45–50mmHg], FiCO2 12% [PaCO2=80–100mmHg]) to inhibit the adverse effects attributable to mechanical overdistension. These protective effects in turn would be mediated by the following mechanisms (Fig. 1):
- 1)
Improved oxygenation, pulmonary elastance and vascular permeability, with histologically manifest improvement of the pulmonary lesions.28,29
- 2)
Prevention of the activation of the MAP-kinases pathway, thereby reducing the production of proinflammatory mediators.30–32
- 3)
Significant reduction of apoptosis, oxidative stress and inflammatory markers as a result of inhibition of the activation of the MAP-kinase and SAPK/JNK pathways at alveolar epithelial cell level.26
- 4)
Decreased inflammatory response and improvement of lung mechanics by inhibiting the canonical NF-κB pathway, the degradation of IkB-alfa and p65 nuclear translocation.25
Immune modulating mechanisms of carbon dioxide at cell level: hypercapnic respiratory acidosis, through the inhibition of ADAM-17, blocks the phosphorylation of P44/P42 induced by pulmonary overdistension, thereby reducing inflammation at alveolar epithelial cell level (A). On the other hand, hypercapnic acidosis stimulates translocation of the ReIB antiinflammatory gene, and possibly reduces the translocation of p65 by inhibiting the canonic NF-kB pathway (B). Hypercapnic acidosis prevents apoptosis produced by mechanical overdistension, by inhibiting the MAPK ASK-1-JNK/p38 pathway, and reducing the levels of ASK-1, p38, JNK and caspase 3 (C). Hypercapnic acidosis delays alveolar edema clearance by inducing endocytosis of the Na+-K+-ATPase pump (D). ADAM-17: ADAM metallopeptidase 17; ASK-1: apoptosis signal-regulating kinase-1; EGFR: epidermal growth factor receptor; ERK: extracellular signal-regulated kinase; MAPK: mitogen-activated protein kinase; NF-kB: nuclear factor kappa B; PKA: protein kinase A. Courtesy of Contreras M. Curr Opin Anesthesiol 2015, 28:26–37.69 Copyright © 2015 Wolters Kluwer Health, Inc. All rights reserved.
At least 50% of all patients that survive ARDS suffer an important decrease in respiratory functional reserve capacity (FRC), with functional limitation and increased morbidity over the long term.33,34 The post-ARDS cell repair process is therefore extremely important in this group of patients.
Hypercapnia delays epithelial and alveolar repair after VILI through the following mechanisms (Fig. 1):
- 1)
Delayed alveolar membrane repair as a result of diminished cell migration dependent upon the NF-κB pathway.35,36
- 2)
Decreased alveolar edema clearance through inhibition of the Na+-K+-ATPase pump mediated by an endocytic process. This phenomenon is independent of the pH and can be activated by signals from cytoskeletal proteins possessing receptors for CO2.37–40
Tissue ischemia–reperfusion damage occurs when oxygenated blood returns to the organ or tissue after a period of ischemia, hypoxia or anoxia. It is characterized by the activation of an inflammatory cascade with the release of cytokines, neutrophils, reactive oxygen species (ROS) and free radicals.41
Such damage occurs in different scenarios in the critically ill patient, such as lung transplantation, pulmonary embolism or ARDS.
Hypercapnic acidosis has been shown to be able to attenuate ischemia-reperfusion damage at pulmonary level through the following mechanisms:
- 1)
By preserving the barrier function of the capillary endothelium, reducing its permeability through a decrease in xanthine-oxidase activity.42
- 2)
By attenuating the inflammatory response, reducing the TNF-α levels in bronchoalveolar lavage and diminishing lipid peroxidation.43–45
- 3)
By inhibiting the NF-κB pathway, reducing inflammation and apoptosis at pulmonary level.46
In experimental models of sepsis, hypercapnia produces a great variety of effects upon the immune system, which in turn influences the level of bacterial growth.
The effects of hypercapnia upon the immune response have been investigated both in vitro and in vivo:
- 1)
Selective inhibition of IL-6 and TNF-α, which are cytokines that play a key role in host defense.47
- 2)
Reduction of phagocytosis mediated by alveolar macrophages in animal models and in humans.47
- 3)
Inhibition of activation of the canonical NF-κB pathway, which promotes the activation of genes implicated in host defense. Such inhibition allows activation of the non-canonical NF-κB pathway, which exerts antiinflammatory and immunosuppressive action.48,49
Hypercapnia has been shown to reduce host defense capacity following aggression of microbial origin. This has been evidenced in a murine model of pneumonia due to Pseudomonas aeruginosa subjected to hypercapnia.50 In this model, the mice exposed to high levels of CO2 showed greater mortality and an increase in the number of colonies of this bacterial species both in the lungs and in other organs. Likewise, a decrease was observed in the levels of IL-6 and TNF-α at pulmonary level, resulting in diminished neutrophil-mediated phagocytic capacity.50
Hypercapnia and the NF-κB pathwayThe NF-κB network is composed of 5 families of protein monomers (p65/RelA, RelB, cREl, p50 and p52) which form homodimers or heterodimers that bind to DNA.
The NF-κB network is regulated via two pathways: canonical and non-canonical. These two pathways control the levels and activation of the NF-κB dimers in response to stimuli, regulating a series of genetic expressions through the recruitment of co-activators or transcription factors.51
Hypercapnia appears to have important effects upon this complex of proteins by inhibiting the activation of protein ReIB via the non-canonical pathway, which stimulates cell repair, proliferation and growth, and would prevent the activation of protein p65 (which is activated via the canonical pathway), which exerts proinflammatory effects52 (Fig. 1). Therefore, CO2 exerts a series of effects upon these pathways at inflammation and alveolar repair level, and in relation to host defense and immunity, as commented above.
Effects of hypercapnia in acute respiratory distress syndromePermissive hypercapniaHickling et al.53 were the first to propose protective ventilation strategies as rescue measure in patients with severe ARDS, with the aim of limiting VILI. These strategies comprised the following elements: (1) reduction of PIP and ventilation with low Vt; (2) application of positive end-expiratory pressure (PEEP); and (3) acceptance of high PaCO2 values. The authors postulated that “an alternative to the mechanical ventilation strategies would be limiting PIP, reducing Vt and allowing the elevation of PaCO2. The latter would stabilize at a new and higher level, and the elimination of CO2 would be maintained at lower levels of alveolar ventilation, as occurs in patients with chronic obstructive pulmonary disease (COPD)”. Although this study presented a series of limitations, the observed great and significant difference in hospital mortality in favor of the protective ventilation and permissive hypercapnia strategies (16% versus 39.6%) gave rise to a series of prospective studies on protective ventilation in patients with ARDS.
Based on these findings, 5 randomized prospective clinical trials were carried out to analyze the effect of protective ventilation in patients with ARDS.54–58 Two of these studies recorded a significant decrease in mortality54,57 with protective ventilation versus ventilation with high Vt (12ml/kg ideal weight) (see Table 4e of supplementary material). Although permissive hypercapnia was present in these studies, there are certain limitations in concluding that CO2 exerts a protective effect, such as the important statistical variability, the non-randomization of patients to normocapnia versus hypercapnia, and the fact that the primary objective of these studies was to demonstrate the effect of ventilation with low tidal volumes (Vt 6ml/kg ideal weight) upon mortality in patients with ARDS.
A secondary analysis of the ARMA study was made with the purpose of determining whether hypercapnic acidosis adds to the effect of protective ventilation strategies with low Vt settings.59 The hypercapnic patients ventilated with Vt 12ml/kg ideal weight were seen to suffer less mortality than those with normal CO2 levels and the same ventilatory pattern. However, in the group of patients ventilated with 6ml/kg ideal weight, no differences in mortality were recorded according to the CO2 levels in plasma. It is therefore difficult to draw firm conclusions as to whether hypercapnia may benefit patients with ARDS beyond the protection afforded by ventilation with low Vt settings.
Recently, Nin et al.,60 in a secondary analysis of three prospective non-interventional cohort studies involving a total of 1899 patients with ARDS, found that those individuals who developed hypercapnia – defined as PaCO2≥50mmHg within the first 48h of mechanical ventilation – presented significantly lower PaO2/FiO2, higher plateau pressure levels, and a significant increase in mortality in the Intensive Care Unit (ICU) (62.5% versus 49.6%; odds ratio [OR]: 1.93; 95% confidence interval [95%CI]: 1.32–2.81; p=0.001). Likewise, the incidence of barotrauma and of renal and cardiovascular dysfunction was greater in the patients with hypercapnia.
These findings are consistent with those published by Tiruvoipati et al.61 In their retrospective study conducted in New Zealand and Australia, involving over 250,000 patients over a 14-year period, a significant increase in mortality was recorded in those patients who within the first 24h of mechanical ventilation developed hypercapnic acidosis (pH<7.35 and PaCO2>45mmHg) (OR: 1.74; 95%CI: 1.62–1.88) and compensated hypercapnia (pH 7.35–7.45 and PaCO2>45mmHg) (OR: 1.18; 95%CI: 1.10–1.26), compared with the patients presenting normocapnia and normal pH (PaCO2 35–45mmHg and pH 7.35–7.45) (p<0.001).
Randomized clinical trials with a more adequate design are still needed to clarify the effect of permissive hypercapnia in patients with acute lung injury.
Alveolar dead spaceIt is important to remember that patients with ARDS have severely altered CO2 clearance due to the increase in alveolar dead space (VDALV). The increase in VDALV in these patients is secondary to alterations of the ventilation/perfusion (V/Q) ratio, with alveoli ventilated out of proportion to the low perfusion they receive (V>Q). This is the result of microcirculatory alterations secondary to endothelial damage, microthrombosis and the accumulation of cell detritus.62
Interest in the study of dead space in ARDS was impulsed by Nuckton et al.24 In a prospective study of 179 patients with moderate-severe ARDS, these authors found the increase in dead space (VD/VT) measured in the first 24h of ARDS to be independently correlated to an increase in mortality risk. The mean VD/VT was 0.54 among the survivors versus VD/VT of 0.63 in those who died as a result of the syndrome. Furthermore, the mortality risk was found to increase 45% for every 0.05 increment in dead space above 0.57. The measurement of dead space was seen to be of greater prognostic value than other measures such as PaO2/FiO2, lung distensibility or the severity of disease.
Extracorporeal elimination of carbon dioxide: a promising futureThe reason for tolerating high CO2 levels is to allow low Vt settings, lower plateau pressures and lesser minute-ventilation values with the purpose of reducing the risk of VILI. Nevertheless, up to 30% of all patients with ARDS present evidence of VILI despite the use of protective ventilation strategies.63
However, allowing the elevation of CO2 in the critical patient with ARDS requires a number of considerations:
- 1)
The clinically acceptable limits in the study of Hickling et al.53 (maximum mean PaCO2 67mmHg, with mean pH 7.20) seem to be reasonable and well tolerated by the patient. However, higher levels of respiratory acidosis may have undesirable effects (cerebral vasodilatation, pulmonary hypertension, arrhythmias).
- 2)
Although beneficial effects of CO2 upon the lung parenchyma have been described, permissive hypercapnia does not resolve the problem of non-perfused regions of the lung with high VD/VT.
- 3)
Hypercapnia is not the best companion for patients with ARDS, who suffer reduced distensibility, hypoxia, dyspnea and high ventilatory demand, and with the need for a degree of sedation to allow the mechanical ventilator to control the patient requirements.
In sum, hypercapnia seems to be more of a last resort option than a routine or therapeutic strategy in patients with ARDS.
Based on the above, extracorporeal CO2 removal (ECCO2R) has been evaluated as an adjuvant to protective ventilation, with the purpose of being able to lower the Vt levels to under 6ml/kg ideal weight – a strategy referred to as “ultraprotective ventilation” – and avoid the potential adverse effects of extreme acidosis levels.
In a study of 32 patients with ARDS for less than 72h, Terragni et al.64 observed a decrease in inflammatory cytokine levels in the bronchoalveolar lavage of those patients subjected to ultraprotective ventilation (Vt close to 4ml/kg ideal weight) plus ECCO2R – this biological effect evidencing lesser VILI.
In the Xtravent study, Bein et al.65 observed no impact in terms of mortality among patients with ARDS subjected to ultraprotective ventilation plus ECCO2R. However, a post hoc analysis of the group of patients with PaO2/FiO2<150 revealed a decrease in the days of mechanical ventilation among the patients subjected to ultraprotective ventilation (Vt 3ml/kg ideal weight plus ECCO2R).
Recently, Taccone66 and the members of the working group of the EuroELSO conducted a systematic review of the available clinical evidence on the use of ECCO2R in the critical patient. The review only included studies with a control group. Six studies were identified for analysis: three referred to chronic obstructive pulmonary disease and three to ARDS. These 6 publications included a total of 279 patients, of which 142 were subjected to ECCO2R with the purpose of providing ultraprotective ventilation. The only two randomized studies corresponded to patients with ARDS. All of the studies showed important heterogeneity of the inclusion criteria, and none of them had enough statistical power to conclude that important clinical effects (e.g., referred to ICU stay or mortality) were obtained.
The SUPERNOVA trial (NCT 02282657), which has ended its first pilot recruitment of patients with moderate ARDS subjected to ultraprotective ventilation plus ECCO2R, will provide more data on the use of ECCO2R in this group of patients. Likewise, a randomized clinical trial is underway, designed to analyze 90-day mortality in patients with hypoxemic acute respiratory failure subjected to ultraprotective ventilation with venovenous ECCO2R (ECCO2R V-V) (NCT 02654327).
To date, the available literature does not allow us to establish clear recommendations on the use of this technique in the critical patient – its application being confined for now to the experimental setting. On the other hand, the difficulties in predicting the progression of ARDS in an early stage may limit the use of ECCO2R in clinical practice.
Should a buffer be used to treat acidosis?The use of buffers to treat hypercapnic acidosis remains a common but controversial clinical practice.
The use of buffers has been justified on the grounds of the physiological effects associated with extreme levels of hypercapnic and metabolic acidosis (pH<7.10). In particular, these effects comprise a decrease in inotropism with hemodynamic instability refractory to catecholamines, actions upon cerebral and immune function, and diminished energy metabolism.
There are doubts regarding the use of sodium bicarbonate – the buffer most commonly employed in clinical practice. Its administration could worsen intracellular acidosis through the generation of CO2, which is produced by the reaction between HCO3− and carbonic anhydrase, and diffuses passively within the cells.
Tromethamine (tris-hydroxy-metyl aminomethane [THAM]) could be regarded as an alternative buffer of choice in cases where hypercapnic acidosis must be treated. Since THAM easily diffuses through the cells, it corrects the pH levels and reduces the CO2 concentrations. In this respect, by correcting the pH levels, THAM could mitigate the adverse effects of acidosis at cardiovascular level, with the recovery of hemodynamic stability.67 However, in addition to the complications associated with its use (irritation, tissue necrosis, hypoglycemia and respiratory depression), THAM is unable to solve the problem of non-perfused lung regions, which result in an increase in VDALV.68
ConclusionsCarbon dioxide is much more than simply metabolic waste: it is a potent biological agent with a range of actions upon cells, and with immune modulating effects at both respiratory and systemic level.
Although preclinical studies indicate a beneficial effect of hypercapnic acidosis in terms of a decrease in ventilator-induced lung injury (VILI), there are also adverse effects as evidenced by clinical studies in which an increase in mortality among ARDS patients has been observed. Further randomized clinical studies are needed to establish the true impact of hypercapnia in these patients.
The use of ECCO2R could be important as an adjuvant strategy in the management of patients with ARDS in the absence of severe hypoxemia, allowing ultraprotective ventilation, reducing the risk of VILI, and controlling the PaCO2 levels.
We consider it important to define ideal PaCO2 levels in order to balance their favorable and unfavorable biological effects.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Morales Quinteros L, Bringué Roque J, Kaufman D, Artigas Raventós A. Importancia del dióxido de carbono en el paciente crítico: implicaciones a nivel celular y clínico. Med Intensiva. 2019;43:234–242.