Extracorporeal membrane oxygenation (ECMO) affords mechanical circulatory assistance associated to high mortality. However, weaning from such mechanical support may not imply improved short- or long-term survival. This study describes the characteristics and evolution of patients with refractory cardiogenic shock (RCS) subjected to venoarterial ECMO (VA-ECMO) in a hospital with a heart transplant program.
DesignA single-center, retrospective cohort study was carried out.
SettingThe cardiovascular ICU of a tertiary hospital.
PatientsForty-six patients consecutively subjected to VA-ECMO over 6 years.
InterventionsHospital mortality after weaning from ECMO and overall survival (OS) were analyzed.
ResultsFifteen patients (33%) died with VA-ECMO and 31 (67%) were weaned after 8 days of support (IQR: 5–15). Fourteen patients under went transplantation. Hospital mortality in these patients was 32% (10/31), and was associated to age (p=0.001), SAPS II score (p=0.009), cannulation bleeding (p=0.01) and post-acute myocardial infarction RCS (p=0.001). After a median follow-up of 27 months (IQR: 11–49), 91% of the patients discharged from hospital were still alive. Overall survival after weaning from assistance was associated to the type of cardiac disease (p=0.002). Patients with RCS after acute myocardial infarction had a poorer prognosis.
ConclusionsIn our experience, VA-ECMO can be used as mechanical assistance in the management of RCS. The technique is associated to high early mortality, though the long-term survival rate after hospital discharge is good.
La membrana de oxigenación extracorpórea (ECMO) es un tipo de asistencia circulatoria que asocia elevada mortalidad. Sin embargo, superar la fase inicial de soporte mecánico no implica supervivencia ni a corto ni a largo plazo. Objetivo: describir las características y evolución de los pacientes con shock cardiogénico refractario (SCR) asistidos con ECMO veno-arterial (ECMO-VA) en un hospital con programa de trasplante cardíaco.
DiseñoEstudio de cohortes y retrospectivo de centro único.
ÁmbitoUCI cardiológica de un hospital terciario.
PacientesUn total de 46 pacientes asistidos consecutivamente con una ECMO-VA durante 6 años.
IntervencionesAnálisis de la mortalidad hospitalaria tras la retirada del soporte mecánico, de la supervivencia global (SG) y de los factores asociados.
ResultadosQuince pacientes (33%) fallecieron con la ECMO-VA y 31 (67%) sobrevivieron a su retirada tras un soporte de 8 días (RIC: 5-15); 14 pacientes fueron trasplantados. La mortalidad hospitalaria en estos pacientes fue del 32% (10/31) y se relacionó con: edad (p=0,001), SAPS-II (p=0,009), sangrado de cánulas (p=0,01), indicación de SCR post-IAM (p=0,001). Con una mediana de seguimiento de 27 meses (RIC: 11-49), seguían vivos el 91% de los pacientes que fueron dados de alta del hospital. La SG tras la retirada de la ECMO-VA se relacionó con el tipo de indicación (p=0,002), teniendo peor pronóstico los pacientes con SCR postinfarto.
ConclusionesEn nuestra experiencia, la ECMO-VA es un tipo de asistencia mecánica que puede utilizarse en el manejo del SCR. Asocia una mortalidad precoz elevada, pero tras superar la fase hospitalaria la supervivencia de los pacientes es buena.
Extracorporeal membrane oxygenation (ECMO) is a circulatory assist method that has been developed from the cardiopulmonary bypass technique.1 It is able to provide prolonged life support (from days to weeks) in patients with refractory cardiogenic shock and/or potentially reversible severe respiratory failure refractory to maximum conventional treatment efforts.2–6 Patients with cardiogenic shock who are treated with vasoactive drugs and inotropic agents, often assisted by an intraaortic balloon pump (IABP), suffer a high mortality rate (between 40 and 60%) when such management measures fail.7,8
In relation to venoarterial ECMO (VA-ECMO), few studies on the experience with this technique in Spain can be found in the literature.9–11 No randomized clinical trials have been published in the international literature, though the existing studies report highly variable mortality rates of between 35 and 70% as determined 30 days after implantation, depending on the indication involved.2,3,12–16 This high early mortality rate among VA-ECMO assisted patients is fundamentally attributable to refractory multiorgan failure (MOF).13 However, surviving the initial mechanical support phase does not necessarily imply survival among patients with refractory cardiogenic shock. Although few studies have examined this issue, the in-hospital mortality rate of patients who have been successfully weaned from ECMO remains high (31–59%).17,18
Lastly, analyzing the long-term patient outcome following acute disease is essential in order to justify the investments made. In fact, long-term survival after serious disease has been identified as an important therapeutic endpoint in assessing the efficacy of new therapies.19–21 In this regard, the studies that have analyzed the long-term outcomes in patients requiring VA-ECMO report one-year survival rates of 26–57%.12–14
The present study was carried out to describe the characteristics and analyze the short-term outcome – measured as 30-day survival and in-hospital mortality – and the long-term outcome in patients with refractory cardiogenic shock subjected to VA-ECMO during a period of 6 years in our tertiary hospital with a heart transplantation program.
Patients and methodsStudy designA retrospective observational study was made of the outcome of a cohort of patients admitted to a cardiological Intensive Care Unit (ICU) due to refractory cardiogenic shock and treated with VA-ECMO during a period of 6 years between January 2010 and December 2015. The patients included in the study were treated according to the protocols of the cardiological ICU, which consists of a medical-surgical Unit with 13 beds that assists patients with severe acute cardiovascular conditions and post-heart surgery cases. The study was approved by the local Clinical Research Ethics Committee.
PatientsThe study included all consecutive adult patients admitted to the cardiological ICU with cardiogenic shock or who developed cardiogenic shock after admission to the Unit, and who required support in the form of VA-ECMO (Maquet CardioHelp®). Refractory cardiogenic shock was defined according to previously published hemodynamic criteria22: systolic blood pressure<90mmHg or mean blood pressure<30mmHg with respect to the basal value, with adequate intravascular volume and evidence of a diminished cardiac index (<1.8l/min/m2 or <2.2l/min/m2 with vasoactive support). The VA-ECMO system was implanted by the heart surgery team, and written informed consent was obtained from all the patients or relatives.
Following implantation of the assist system, one subgroup of patients failed to survive the initial mechanical support phase and died with the device. In contrast, other patients showed a favorable clinical course that allowed the suspension of VA-ECMO after recovery of heart function, following heart transplantation, or after placement of some other assist device. The study flowchart is shown in Fig. 1. The patients were analyzed according to clinical outcome. In a first phase we compared the subgroup of patients that died with the device versus those that were able to recover heart function. This was followed by a second study of those patients that survived VA-ECMO, and in which we analyzed in-hospital evolution and long-term outcome. The follow-up of all patients discharged from hospital continued up until 30 March 2016.
Study variablesThe study data were obtained from the electronic case histories and hospital intranet (internal electronic network of the hospital), and were entered in a MS Access 2007® database.
The primary endpoints were in-hospital mortality and overall survival, estimated as the time from VA-ECMO implantation to death of any cause. The following variables were recorded: a) referred to the patient: age, sex, comorbidity (diabetes mellitus, arterial hypertension, dyslipidemia, atrial fibrillation, chronic ischemic heart disease, chronic obstructive pulmonary disease [COPD] and chronic renal failure), body surface, left ventricular ejection fraction (LVEF) prior to implantation of the device and LVEF after implantation (before hospital discharge); b) referred to patient management: type of access (central or peripheral); type of indication13 (acute myocardial infarction, non-ischemic acute myocardiopathy [MCP], post-cardiotomy, end-stage dilated MCP–including idiopathic dilated MCP, valvular MCP, chronic ischemic MCP and due to anthracycline toxicity–acute graft failure, and miscellaneous); days of assist; implantation in cardiac arrest (CA); need for intraaortic balloon pump before VA-ECMO; percutaneous coronary intervention (PCI); weaning or withdrawal of assist following the recovery of heart function; need for heart transplantation; and need for support with some other ventricular assist device (Levitronix® Centrimag); c) severity and outcome parameters: Acute Physiology and Chronic Health Evaluation II (APACHE-II) score; Simplified Acute Physiology Score II (SAPS-II); lactate concentration prior to implantation (in mmol/l); lactate concentration 24h after implantation; d) complications: major bleeding of the cannulas (requiring the transfusion of two or more packed red cell units in 24h, or revision surgery); lower limb ischemia; cerebral hemorrhage; acute renal failure defined according to the AKIN scale23; nosocomial infection (developing from 48h after implantation and including ventilator associated pneumonia, bacteremia secondary to catheter or device, soft tissue infection and urinary infection); need for renal replacement therapy (RRT); need for tracheostomy; and days of mechanical ventilation (MV); and e) evolutive parameters: days of stay in the ICU and days in hospital; condition at discharge (alive/deceased); date of last hospital visit and condition (alive/deceased), and cause of death (refractory MOF, septic shock, severe brain damage and other causes).
Statistical analysisThe data were analyzed using the SPSS version 18 statistical package. Quantitative variables were reported as the mean and standard deviation (SD) or as the median and interquartile range (IQR), while qualitative variables were reported as numbers and percentages. The comparison of quantitative variables was carried out using the Student t-test for independent samples in the presence of a normal distribution, and the Mann–Whitney U-test in the case of a non-normal distribution. Qualitative variables in turn were compared with the chi-squared test or Fisher exact test, as applicable. Normal data distribution was assessed using the Kolmogorov–Smirnov test. The multivariate analysis was based on a binary logistic regression model (forward conditional method) to study the factors associated to in-hospital mortality, with the inclusion of all those variables yielding p<0.2 in the univariate analysis. Overall survival was evaluated using Kaplan–Meier curves, estimating the proportion of patients still alive during follow-up, which ended on 30 March 2016. Statistical significance was considered for p<0.05.
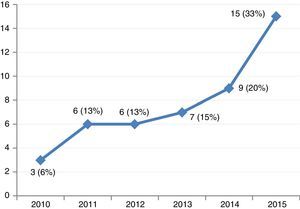
ResultsThe study included 46 patients requiring mechanical support with VA-ECMO due to cardiogenic shock between January 2010 and December 2015. During this period, the use of devices of this kind increased gradually in our center (Fig. 2). A peripheral access was used in 39 cases (85%), and a percutaneous approach was adopted in all patients. The indications were: 1) end-stage dilated MCP (13 patients, 28%), including 6 cases of idiopathic dilated MCP, three cases of chronic ischemic MCP, two cases of valvular MCP, and two cases due to anthracycline toxicity; 2) acute myocardial infarction (10 patients, 21%); 3) post-cardiotomy (8 patients, 17%); 4) acute graft failure (7 patients, 15%); 5) non-ischemic acute MCP (6 patients, 13%), and 6) miscellaneous (2 patients, 4%). The patient characteristics are shown in Table 1.
Characteristics of all the patients and comparison between those who recovered heart function and those who died with VA-ECMO.
| All patients (n=46) | Died with VA-ECMO (n=15) | Recover heart function (n=16) | p-valuea | |
|---|---|---|---|---|
| Age, years | 48±15 | 51±14 | 52±16 | 0.8 |
| Males | 28 (61) | 7 (47%) | 10 (63%) | 0.5 |
| Body surface, m2 | 1.82±0.2 | 1.83±0.3 | 1.78±0.2 | 0.6 |
| Previous LVEF, % | 22±14 | 19±13 | 28±17 | 0.15 |
| Peripheral access | 39 (85) | 12 (80) | 12 (75) | 0.5 |
| IABP before VA-ECMO | 23 (50) | 7 (47) | 5 (31) | 0.4 |
| Implanted in cardiac arrest | 5 (11) | 2 (13) | 2 (12) | 0.9 |
| Comorbidity | ||||
| Diabetes mellitus | 8 (17) | 1 (7) | 5 (31) | 0.2 |
| Arterial hypertension | 14 (30) | 3 (20) | 7 (44) | 0.2 |
| Dyslipidemia | 10 (22) | 1 (7) | 5 (31) | 0.2 |
| Atrial fibrillation | 5 (11) | 0 | 2 (13) | 0.5 |
| Chronic ischemic heart disease | 8 (17) | 2 (13) | 1 (6) | 0.6 |
| COPD | 2 (4) | 0 | 1 (6) | 0.9 |
| Chronic renal failure | 4 (9) | 2 (13) | 2 (12) | 0.9 |
| Severity and prognostic endpoints | ||||
| APACHE-II | 14±7 | 15±7 | 15±7 | 0.8 |
| SAPS-II | 40±13 | 42±10 | 40±16 | 0.6 |
| Lactate concentration before implantation, mmol/l | 2.6 (1.4–8.7) | 2.6 (1–14.4) | 2.6 (1.9–5.5) | 0.9 |
| Lactate concentration after 24h, mmol/l | 1.5 (0.9–3.4) | 1.6 (1–7.1) | 1 (0.9–2.5) | 0.6 |
| Indication | ||||
| Acute myocardial infarction | 10 (21) | 2 (13) | 4 (25) | 0.4 |
| Non-ischemic acute MCP | 6 (13) | 2 (13) | 3 (19) | 0.6 |
| End-stage dilated MCP | 13 (28) | 4 (27) | 0 | 0.03 |
| Post-cardiotomy | 8 (17) | 2 (13) | 5 (31) | 0.2 |
| Acute graft failure | 7 (15) | 3 (20) | 4 (25) | 0.7 |
| Miscellaneous | 2 (4) | 2 (13) | 0 | 0.1 |
| Evolution | ||||
| Limb ischemia | 13 (28) | 4 (27) | 3 (19) | 0.7 |
| Bleeding of cannulas | 13 (28) | 4 (27) | 5 (31) | 0.9 |
| Cerebral hemorrhage | 4 (9) | 2 (13) | 1 (6) | 0.6 |
| Renal failure | 27 (59) | 8 (53) | 12 (75) | 0.3 |
| Renal replacement techniques | 18 (39) | 4 (27) | 9 (56) | 0.1 |
| Tracheostomy | 14 (30) | 0 | 7 (44) | 0.007 |
| Nosocomial infection | 24 (52) | 4 (27) | 12 (75) | 0.01 |
| Days on VA-ECMO | 8 (5–15) | 7 (2–14) | 7 (5–10) | 0.8 |
| Days on mechanical ventilation | 17 (8–29) | 8 (2–19) | 20 (13–33) | 0.05 |
| Days in ICU | 20 (9–35) | 8 (2–18) | 23 (17–33) | 0.007 |
| Days in hospital | 43 (23–78) | 21 (9–39) | 51 (26–84) | 0.05 |
The results are expressed as mean±SD, median (IQR) or number (percentage).
APACHE-II: Acute Physiology and Chronic Health Evaluation II; IABP: intraaortic balloon pump; VA-ECMO: venoarterial extracorporeal membrane oxygenation; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; MCP: myocardiopathy; SPAS-II: Simplified Acute Physiology Score II.
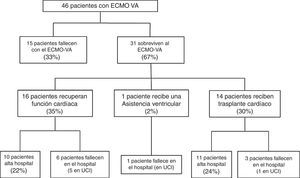
Fifteen patients (33%) died during the mechanical support phase, and 31 patients (67%) survived after a median assist duration of 8 days (IQR: 5–15). In relation to the survivors, mechanical support was withdrawn after the recovery of heart function in 16 cases; after heart transplantation in 14 cases; and after placement of a left ventricular assist device (Levitronix® Centrimag) in one patient (Fig. 1). The characteristics of the patients that failed to improve and died with VA-ECMO, and the characteristics of those that recovered heart function, are compared in Table 1. There were no differences between the two groups capable of predicting success in weaning or the recovery of heart function.
The mortality rate in the ICU was 48% (22/46), while the in-hospital mortality rate was 54% (25/46). The causes of death in the global cohort were: refractory MOF in 12 patients (48%), septic shock in 5 (20%), severe brain damage in 5 (20%), and other causes in three patients (12%). Of the 31 patients that survived weaning from VA-ECMO, 68% (21/31) were discharged from hospital. The LVEF at discharge was 54±12% in the patients that recovered heart function, versus 68±7% in the transplant patients. The characteristics of these 31 patients are compared according to in-hospital evolution in Table 2. The univariate analysis showed that the patients who died in hospital following withdrawal of the device were comparatively older (60±8 vs 41±15; p=0.001), with a higher SAPS-II score at the time of implantation (17±8 vs 12±7; p=0.09), and with a greater incidence of bleeding of the cannulas during the mechanical support phase (60% vs 14%; p=0.01). In addition, these patients had been assisted more often due to post-infarction cardiogenic shock (60% vs 9%; p=0.004). In the multivariate analysis, age was the only independent variable correlated to poorer in-hospital evolution in these patients (OR 1.2; 95%CI: 1.03–1.3).
Differences between the patients discharged from hospital and those who died after withdrawal of VA-ECMO.
| Hospital discharge (n=21) | Died in hospital (n=10) | p-value | |
|---|---|---|---|
| Age, years | 41±15 | 60±8 | 0.001a |
| Males | 14 (67%) | 7 (70%) | 0.9 |
| Body surface, m2 | 1.8±0.2 | 1.83±0.2 | 0.7 |
| Previous LVEF, % | 23±16 | 22±7 | 0.9 |
| Peripheral access | 18 (86) | 9 (90) | 0.9 |
| IABP before VA-ECMO | 11 (52) | 5 (50) | 0.9 |
| Implanted in cardiac arrest | 2 (9.5) | 1 (10) | 0.9 |
| Subjected to heart transplantation | 11 (52) | 3 (30) | 0.2 |
| Comorbidity | |||
| Diabetes mellitus | 3 (14) | 4 (40) | 0.1a |
| Arterial hypertension | 5 (24) | 6 (60) | 0.09a |
| Dyslipidemia | 4 (19) | 5 (50) | 0.09a |
| Atrial fibrillation | 4 (19) | 1 (10) | 0.9 |
| Chronic ischemic heart disease | 3 (14) | 3 (30) | 0.3 |
| COPD | 1 (5) | 1 (10) | 0.9 |
| Chronic renal failure | 1 (5) | 1 (10) | 0.9 |
| Severity and prognostic endpoints | |||
| APACHE-II | 12±7 | 17±8 | 0.09a |
| SAPS-II | 34±11 | 48±15 | 0.009a |
| Lactate concentration before implantation, mmol/l | 2.8 (2–9.4) | 2 (1.4–2.9) | 0.4 |
| Lactate concentration after 24h, mmol/l | 1.2 (1–9.4) | 1 (0.75–2.4) | 0.6 |
| Indication | |||
| Acute myocardial infarction | 2 (9) | 6 (60) | 0.004a |
| Non-ischemic acute MCP | 4 (19) | 0 | 0.2 |
| End-stage dilated MCP | 7 (33) | 2 (20) | 0.5 |
| Post-cardiotomy | 6 (29) | 0 | 0.07a |
| Acute graft failure | 2 (9) | 2 (20) | 0.4 |
| Evolution | |||
| Limb ischemia | 5 (24) | 4 (40) | 0.4 |
| Bleeding of cannulas | 3 (14) | 6 (60) | 0.01a |
| Cerebral hemorrhage | 2 (9) | 0 | 0.9 |
| Renal failure | 12 (57) | 7 (70) | 0.7 |
| Renal replacement techniques | 8 (38) | 6 (60) | 0.4 |
| Tracheostomy | 8 (38) | 6 (60) | 0.4 |
| Nosocomial infection | 13 (62) | 7 (70) | 0.9 |
| Days on VA-ECMO | 8 (6–13) | 7 (4–16) | 0.7 |
| Days on mechanical ventilation | 22 (16–36) | 17 (9–51) | 0.7 |
| Days in ICU | 30 (21–44) | 19 (8–43) | 0.4 |
| Days in hospital | 66 (46–90) | 38 (18–86) | 0.7 |
| Multivariate analysis | |||
| Odds ratio | 95%CI | p-value | |
| Age | 1.2 | 1.03–1.3 | 0.02 |
| SAPS-II | 1.1 | 0.97–1.2 | 0.2 |
| Post-acute infarction shock | 5.9 | 0.3–116.2 | 0.2 |
The results are expressed as mean±SD, median (IQR) or number (percentage).
APACHE-II: Acute Physiology and Chronic Health Evaluation II; IABP: intraaortic balloon pump; VA-ECMO: venoarterial extracorporeal membrane oxygenation; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; MCP: myocardiopathy; SPAS-II: Simplified Acute Physiology Score II.
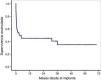
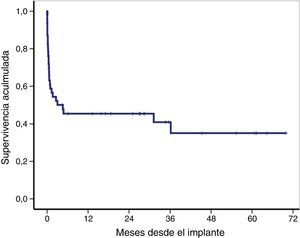
The overall survival of the global cohort following implantation of VA-ECMO is shown in Fig. 3. Overall survival after 30 days and 12 and 36 months was 59%, 46% and 41%, respectively. Twenty-one patients (46%) were discharged from hospital, with a median follow-up after discharge of 27 months (IQR: 11–49). Ninety-one percent of the patients (19/21) were still alive at last follow-up.
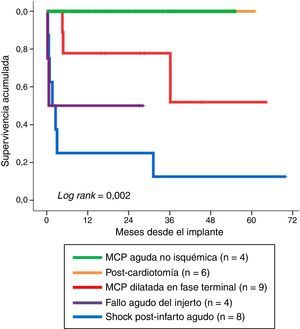
Fig. 4 shows long-term survival according to the type of indication in those patients who survived the mechanical support phase. A significant finding (log rank: 0.002) was that the least favorable evolution corresponded to the patients in which mechanical support was indicated due to post-infarction refractory cardiogenic shock–with a survival rate at last follow-up of 12.5% (1/8). In contrast, the evolution was more favorable when the indications were acute graft failure and end-stage dilated MCP, with survival rates at last follow-up of 50% (2/4) and 67% (6/9), respectively. The most favorable outcome corresponded to the patients in which mechanical support was indicated due to non-ischemic acute MCP and post-cardiotomy shock: all of these subjects were still alive at the time of VA-ECMO withdrawal.
Post-acute myocardial infarction cardiogenic shockDue to the poorer prognosis of the patients in which mechanical support was indicated due to post-infarction cardiogenic shock, these 10 individuals were analyzed independently. In 8 patients (80%) percutaneous coronary intervention was attempted (with success in 62.5% of the cases); only one patient received VA-ECMO assist before PCI. The survival rate after 30 days was 60%, but only two patients (20%) were discharged from hospital (63% died due to refractory MOF). The characteristics of these patients and their comparison with the rest of the subjects are shown in Table 3.
Differences between patients subjected to mechanical support due to refractory cardiogenic shock secondary to acute myocardial infarction and the rest of indications.
| Post-infarction (n=10) | Other indications (n=36) | p-value | |
|---|---|---|---|
| Age, years | 58±7.8 | 45±16 | 0.001 |
| Males | 5 (50) | 23 (64) | 0.4 |
| Previous LVEF, % | 17±4.4 | 23±15 | 0.07 |
| Peripheral access | 10 (100) | 29 (81) | 0.3 |
| Body surface, m2 | 1.76±0.2 | 1.82±0.2 | 0.4 |
| Implanted in cardiac arrest | 0 | 5 (14) | 0.6 |
| IABP before VA-ECMO | 6 (60) | 17 (47) | 0.7 |
| Subjected to heart transplantation | 3 (30) | 11 (31) | 0.9 |
| Comorbidity | |||
| Diabetes mellitus | 3 (30) | 5 (14) | 0.3 |
| Arterial hypertension | 5 (50) | 9 (25) | 0.2 |
| Dyslipidemia | 4 (40) | 6 (17) | 0.2 |
| Atrial fibrillation | 0 | 5 (14) | 0.6 |
| Chronic ischemic heart disease | 2 (20) | 6 (17) | 0.9 |
| COPD | 0 | 2 (6) | 0.9 |
| Chronic renal failure | 1 (10) | 3 (8) | 0.9 |
| Severity and prognostic endpoints | |||
| APACHE-II | 13±6.5 | 14±7.6 | 0.6 |
| SAPS-II | 44±14 | 39±12 | 0.3 |
| Lactate concentration before implantation, in mmol/l | 2.7 (1.9–9) | 2.3 (1.1–8.8) | 0.7 |
| Lactate concentration after 24 h, in mmol/l | 0.9 (0.6–7.8) | 1.5 (1–3.5) | 0.9 |
| Evolution | |||
| Limb ischemia | 7 (70) | 6 (17) | 0.002 |
| Bleeding of cannulas | 4 (40) | 9 (25) | 0.4 |
| Cerebral hemorrhage | 0 | 4 (11) | 0.6 |
| Renal failure | 6 (60) | 21 (58) | 0.9 |
| Renal replacement therapy | 4 (40) | 14 (39) | 0.9 |
| Tracheostomy | 6 (60) | 8 (22) | 0.05 |
| Nosocomial infection | 7 (70) | 17 (47) | 0.2 |
| Days on VA-ECMO | 16 (9–27) | 7 (5–9) | 0.01 |
| Days on mechanical ventilation | 45 (18–59) | 15 (6–22) | 0.07 |
| Days in ICU | 31 (18–49) | 18 (7–33) | 0.3 |
| Days in hospital | 48 (24–70) | 41 (18–84) | 0.7 |
| ICU mortality | 6 (60) | 16 (44) | 0.5 |
| In-hospital mortality | 8 (80) | 17 (47) | 0.06 |
The results are expressed as mean±SD, median (IQR) or number (percentage).
APACHE-II: Acute Physiology and Chronic Health Evaluation II; IABP: intraaortic balloon pump; VA-ECMO: venoarterial extracorporeal membrane oxygenation; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; MCP: myocardiopathy; SPAS-II: Simplified Acute Physiology Score II.
In our center, mechanical support with VA-ECMO is increasingly used for the management of refractory cardiogenic shock. The acute mortality rate of our patients was high, though it must be underscored that the patients who survived beyond admission to hospital had an excellent survival rate of 91% with a median follow-up of 27 months, and with heart function close to normal. These results suggest that circulatory assist of this kind could rescue and offer chances for long-term survival among patients with refractory cardiogenic shock, including some cases characterized by circulatory arrest.
In our series, almost 70% of the patients subjected to VA-ECMO survived the initial phase of mechanical support. On comparing these results with those obtained in other series involving at least 35 patients and likewise including refractory cardiogenic shock of different origins, the data are seen to be similar – with 38–70% of the patients being found to survive the mechanical support phase.12,13,17,18,24 In our study, none of the analyzed variables were of help in predicting successful weaning or the recovery of heart function. However, other studies have correlated echocardiographic,25 laboratory test13,26 and clinical parameters26 to the prediction of weaning. The lack of results in our series may be due to both the limited number of patients involved and the study design, which was not intended to examine this aspect.
With regard to the short-term prognosis, our results are similar to those of other series. The survival rate 30 days after VA-ECMO implantation documented in the literature varies between 30 and 65%, depending on the type of indication2,12,14–16,25 – the figure in our mixed series being 59%. With regard to in-hospital mortality, which in our series was 54%, the literature offers highly variable rates in such patients (35–72%)12,13,16,18 – the main causes of death being refractory MOF, severe neurological damage and infection, in concordance with our own observations.13,18,27 In our opinion, it is more important to analyze patient evolution beyond VA-ECMO, since the withdrawal of mechanical support does not necessarily imply survival. In fact, up to one-third of our patients who survived the mechanical support phase subsequently died in the course of the rest of hospital admission. Few studies have analyzed the evolution of this subgroup of patients, with reported in-hospital mortality rates after the withdrawal of VA-ECMO ranging between 31 and 59%.17,18 In our series, the factors associated to poorer prognosis after withdrawal of mechanical support were a higher SAPS-II score, older age, bleeding of the cannulas and the indication of circulatory assist due to shock following acute myocardial infarction. Specifically, age was the only independent factor associated to greater in-hospital mortality in the multivariate analysis. Previous studies have also related the prognosis of these patients to age,18,28,29 organ damage13,28,29 and the etiology of cardiogenic shock,13,18,28 among other factors.
This is the first Spanish study to report on the long-term evolution of patients assisted with VA-ECMO. Almost one-half of the assisted patients were still alive after one year, and a little over 40% were still alive after three years of follow-up. In comparison, the one-year survival rates found in the literature vary between 26 and 57%, depending on the series.12–14,27 The type of heart disease giving rise to the need for mechanical support was related to long-term survival of the patients after weaning. The outcome of the patients assisted due to post-infarction cardiogenic shock was particularly poor. The survival rate after 30 days in this subgroup of individuals was similar to the 47% recorded in a recent study of 138 patients assisted following acute myocardial infarction.29 However, although 30% underwent heart transplantation, the in-hospital evolution of our patients was poorer than that of another Spanish series reporting an in-hospital survival rate of 72.7% among patients assisted with ECMO due to post-infarction refractory cardiogenic shock.30 In our series, this patient subgroup was small and precluded the drawing of conclusions, though the organ damage caused by initial cardiogenic shock may have played a relevant role in the poor subsequent clinical course. Furthermore, with regard to the rest of the patients, those assisted after acute infarction were older, had a greater incidence of limb ischemia, and were significantly longer on mechanical support. Although our results are unable to provide confirmation, these factors may have influenced the poorer outcome.
The present study has several limitations. Firstly, mention must be made of its retrospective design and the limited number of patients. Nevertheless, the series includes all the VA-ECMO assisted patients in our center, and practically all the study variables were available from the electronic case histories in our center. Secondly, the study involves a population of patients with refractory cardiogenic shock of different origins. Lastly, the single-center nature of the study may complicate extrapolation of the results to other centers. Nevertheless, this is one of the largest Spanish series published to date. Future multicenter studies would be advisable in order to strengthen the clinical relevance of the results and allow a detailed analysis of each of the specific subpopulations of patients that develop cardiogenic shock with an indication of VA-ECMO.
ConclusionsBased on our experience it can be concluded that VA-ECMO is a mechanical support strategy that may be used for the management of patients with cardiogenic shock refractory to conventional therapies. Despite the associated high early mortality rate, long-term survival after the in-hospital phase is good. This type of device not only affords immediate cardiovascular support but can be used as bridging treatment for heart transplantation and – in selected cases – for the implantation of middle-term assist strategies. Patient age and the type of heart disease indicating mechanical support were the factors with the greatest impact upon patient survival in our series.
AuthorshipRenata García-Gigorro was in charge of the literature review, data collection and drafting of the article.
Emilio Renes-Carreño contributed to the study conception and design, and to the analysis and interpretation of the data. He also participated in final critical review of the article.
José Luis Pérez-Vela contributed to data collection and critical review of the article.
Helena Marín-Mateos participated in data collection.
Julián Gutiérrez participated in drafting of the article.
María Angélica Corrés-Peiretti participated in critical review of the article.
Juan Francisco Delgado participated in critical review of the article.
Enrique Pérez de la Sota participated in critical review of the article.
José María Cortina-Romero was in charge of the provision of resources.
Juan Carlos Montejo was responsible for the provision of resources needed to carry out the present study. He likewise gave definitive approval of the presented manuscript version.
Conflicts of interestThe authors declare that they have no conflicts of interest in relation to this study, which has not received financial support.
Please cite this article as: García-Gigorro R, Renes-Carreño E, Pérez-Vela JL, Marín-Mateos H, Gutiérrez J, Corrés-Peiretti MA, et al. Soporte mecánico con membrana de oxigenación extracorpórea veno-arterial (ECMO-VA): evolución a corto y a largo plazo tras la retirada de la asistencia. Med Intensiva. 2017;41:513–522.