Multiple interventions are performed in critical patients admitted to Intensive Care Units (ICUs). This study explores the presence in the daily practice of ICUs of elements related to the 6 bioethics quality indicators of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units, and the participation of their members in the hospital ethics committees.
Materials and methodsA multicenter observational study was carried out, using a survey exploring descriptive aspects of the ICUs, with 25 questions related to bioethics quality indicators, and assessing the participation of ICU members in the hospital ethics committees. The ICUs were classified by size (larger or smaller than 10 beds) and type of hospital (public/private-public concerted center, with/without teaching).
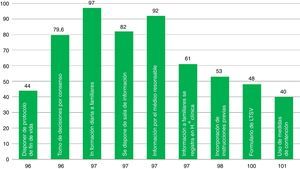
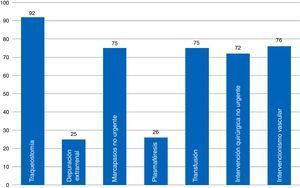
ResultsThe 68 analyzed surveys revealed: daily informing of the family (97%), carried out in the information room (82%); end-of-life care protocols (44%); life support limitation form (48.43%); and physical containment protocol (40%). Compliance with the informed consent process referred to different procedures is: tracheostomy (92%), vascular procedures (76%), and extrarenal clearance (25%). The presence of ICU members in the hospital ethics committee is currently frequent (69%).
ConclusionsInformation supplied to relatives is adequate, although there are ICUs without an information room. Compliance with the informed consent requirements of various procedures is insufficient. The participation of ICU members in the hospital ethics committees is frequent. The results obtained suggest a chance for improvement in the bioethical quality of the ICU.
En los servicios de Medicina Intensiva (SMI) se realizan múltiples intervenciones a los pacientes críticos. Nuestros objetivos son conocer la presencia en la práctica diaria de los SMI de elementos relacionados con los 6 indicadores de calidad en Bioética de la Sociedad Española de Medicina Intensiva Crítica y Unidades Coronarias y la participación de sus miembros en los comités de ética asistencial.
Material y métodosEstudio observacional multicéntrico mediante encuesta que estudia aspectos descriptivos de los SMI, plantea 25 cuestiones relacionadas con los indicadores de calidad bioéticos y describe la participación de miembros del SMI en los comités de ética asistencial. Los SMI se clasifican por tamaño (mayor/menor de 10 camas) y tipo de hospital (público/privado-concertado, docente/no docente).
ResultadosEn las 68 encuestas analizadas encontramos: información familiar diaria (97%), efectuada en sala de información (82%); protocolos de cuidados al final de vida (44%); formulario de limitación de tratamiento de soporte vital (48,43%); protocolo de contención (40%). El cumplimiento del proceso de consentimiento informado es: traqueostomía (92%), intervención vascular (76%), depuración extrarrenal (25%). La presencia actual de miembros del SMI en el Comité de Ética es frecuente (69%).
ConclusionesLa información a familiares es adecuada, aunque hay SMI sin sala de información. El cumplimiento del proceso de consentimiento informado de varios procedimientos es insuficiente. La participación de miembros del SMI en los comités de ética asistencial es frecuente. Los resultados evidencian margen de mejora en la calidad bioética de los SMI.
Intensive Care Units (ICU) are organizations of healthcare professionals providing multidisciplinary care to patients who are eligible for recovery and require organ or support systems.1 In this setting, the medical team's constant preoccupation of providing quality care and implementing continuous improvement cycles should be observed in our daily practice.
The bioethical aspects in the management of critical patients are especially relevant if we want to guarantee quality healthcare. Respect for the patient's autonomy via providing necessary information; the requests for informed consent documents; and the search for previous instructions are ethical and legal prerequisites, and an essential part of the decision-making process. To this end, it is necessary that families and patients are adequately and effectively informed in a comfortable environment in order to preserve privacy. Also, the existence of adapted protocols for end-of-life care, the limitation of life-sustaining treatment (LLST), or the implementation of containment measures allows us to minimize the variability of clinical practice and facilitate the job of the ICU personnel. Eventually all this leads to quality end-of-life care.
The limitation of life-sustaining treatment (LLST) is something common and variable in the ICUs and was implemented in 10 per cent of the patients hospitalized in European intensive care units (ICU) between 1999 and 2000,2 and in 34–41 per cent in some series in our country,3,4 or even up to 70 per cent if there are records of multiple organ failure (MOP).5 A recent series from Spanish ICUs6 shows LLSTs in 34.3 per cent of long-stay patients with serious complications, a 82.7 per cent mortality rate in the ICUs, and a 93 per cent during the hospital stays. The adequation of end-of-life care after deciding to implement the LLST should be taken into consideration in the healthcare process of these patients.
Quality indicators are measuring tools that show the presence or intensity of a given phenomenon with the goal of trying to identify problems or situations of potential improvement.
The use of quality indicators has proven its utility as a tool to measure the routine medical practice and assess the effectiveness of the indicators implemented for quality improvement purposes, which in turn allows the identification and dissemination of the best practices.7 The initial project was called “Quality Indicators in Critical Patients” and has been elaborated by the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) under methodological support from the Avedis Donabedian Foundation.8 The goal of this project was to develop 120 keys indicators for the management of critical patients.9 Back in 2011 these quality indicators were reviewed in a document published by the SEMICYUC10–the review used in the study. The SEMICYUC Task Force on Bioethics (TFB) elaborated six (6) quality indicators (Table 1).
SEMICYUC quality indicators in bioethics.
| Indicator #96. Adequacy of end-of-life-care | Percentage of patients dead in ICUs with LLST in whom the end-of-life care protocol was implemented |
| Indicator #97. Briefing the families of ICU patients | Percentage of pieces of information that abide by the prerequisites |
| Indicator #98. Implementation of previous instructions in the decision-making process | Percentage of incompetent patients in whom there is certainty of the presence of a document with previous instructions |
| Indicator #99. Filling out the informed consent document | Percentage of documents filled out |
| Indicator #100. Limitation of life-sustaining treatment | Percentage LLST indications that abide by the prerequisites |
| Indicator #101. Use of containment measures | Percentage of containment measures adjusted to the protocol |
LLST: limitation of life-sustaining treatment; ICU: Intensive Care Unit.
The main goal of our study was to assess whether in the ICUs routine practice, the aforementioned six SEMICYUC quality indicators included in the section of bioethics are commonly used. Two (2) were the secondary goals: first, assess the presence of these indicators in the ICU while taking into consideration the type and size of hospital we are talking about, the size of the ICU, and the presence of an ICU resident; and second, know the degree of participation of ICU personnel in the hospital healthcare ethics committee (HEC).
Materials and methodsMulticenter observational study conducted through one survey elaborated by the SEMICYUC TFB (Annex 1, material available online) including descriptive-demographic elements from the ICUs such as: autonomous society of Intensive Medicine Care; type of public/mixed-private hospital; with/without university teaching; number of beds in the ICU/total number of beds in the hospital; number of residents and nursing staff working at the ICU. It also includes questions to know the degree of ICU sensitivity to bioethical aspects, the assessment of the presence of elements associated with the aforementioned quality indicators on bioethics in the daily practice, but omitting the degree of compliance with these indicators as described by the 2011 list. Finally, the participation of ICU personnel in their respective hospital HECs was assessed too.
The survey was conducted in two (2) different stages: one initial survey conducted by the Valencian Society of Intensive Care Medicine during the month of February 2015; and a second survey, this time nationwide, conducted during the months of October and December 2015. It was considered as a matter of interest that the SEMICYUC TFB would conduct one survey, and that such survey would be filled out by every ICU, whether by whoever coordinates all bioethical aspects or by the chief of staff; the identity of whoever filled out the survey was not collected. Results were made public by members from the SEMICYUC TFB through their corresponding autonomous societies. The ICUs that were not associated with the SEMICYUC TFB were addressed too through emails and phone calls to the presidency or the secretary of the corresponding intensive care medicine autonomous society.
An aggregate analysis of data was conducted, and the confidentiality of the participating hospitals and professionals was kept in secret. One first global description of all surveys was conducted. Secondly, one subgroup analysis was conducted based on baseline variables that, a priori, may have a higher or lower influence on the sensitivity shown toward bioethical issues: type of hospital (public vs private-mixed) of larger (≥300 beds) or smaller volume (<300); ICU of larger (≥10 beds) or smaller volume (<10) with presence, or not, of intensive care medicine residents.
The categorical variables were described using proportions (per cent), and the continuous numerical variables were expressed as mean, standard deviation, minimum, and maximum. The chi-square test was used to compare the categorical variables. These estimates were conducting using the statistical package for the social sciences SPSS v. 15.
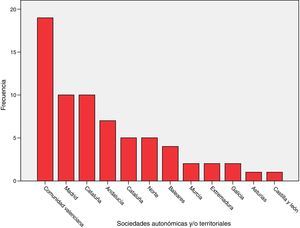
ResultsSixty-eight (68) surveys from other ICUs were included in the analysis (Annex 2, material available online). The distribution based on autonomous societies is shown in Fig. 1. They correspond to ICUs of public (54), mixed (7) and private (7) hospitals.
There is a wide variety in the size of an ICU (from 5 to 48 beds – mean=16.3 and standard deviation=9.6) and in the size of a hospital (from 60 to 1104 beds – mean=426.6 and standard deviation=253.7). Here we are talking about ICUs with less than 10 beds (18) and with 10 or more beds (50) located in 25 hospitals with, at least, 300 beds, and in 43 hospitals with over 300 beds. The training and teaching that takes place in these ICUs is very important: in 63 ICUs, there is training in medicine or nursing (92.6 per cent), and in 48 ICUs there is training in both fields (70.6 per cent); 42 ICUs train residents in intensive care medicine (61.8 per cent), and residents rotate from other medical fields different from intensive medicine in 55 ICUs (80.9 per cent).
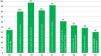
The presence of elements associated with ICU quality indicators is variable (Fig. 2). Aspects such as the decision-making process agreed by the medical staff and the nursing staff, the daily information disclosed to the families, the existence of rooms available to brief the families, and the fact that the information is provided by the doctor treating the patient take place in around 80 per cent of the cases. Other aspects included in the survey such as having end-of-life care protocols, containment measures, or LLST forms available in the ICU are less common (61–40 per cent of the cases). The presence of a member (doctor and/or nurse) from the ICU team in the hospital HEC is a little more common (69 per cent of the cases).
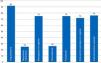
When it comes to indicator #99, the one that has to do with informed consent documents (ICD) recommended by the SEMICYUC TFB and a common player in ICUs (Fig. 3), we found that most ICUs that responded to the survey had available ICDs for percutaneous tracheostomies. Common procedures performed in the ICU such as blood transfusions, non-emergent implantation of pacemakers, non-emergency surgical procedures, and vascular interventional procedures have available ICDs in fewer ICUs (72–76 per cent). Some procedures have a low ICD implementation such as plasmapheresis procedures (usually present in very few ICUs), and extrarenal clearance techniques. Here is a list of some other procedures that are common to ICUs: cannulation for central accesses (No.=16; 23.5 per cent); scheduled electrical cardioversions (No.=9; 13.2 per cent); scheduled sedations for certain procedures (No.=7; 10.3 per cent); thrombolyses in stroke (No.=5; 7.4 per cent). Twenty-eight point six (28.6) per cent of all ICUs (No.=19) have a generic ICD available specifying the procedure that will be performed.
Several subgroup analyses have been conducted to assess the association between the presence of some factors and the frequency of elements associated with the quality indicators. On the tables, the statistically significant results or marginally significant results (p<0.2) are shown together with clinically significant proportional differences. In public hospitals (Table 2), end-of-life care protocols and the filling out of tracheostomy ICDs are more common, while in private hospitals, the presence of protocols of containment measures is more common, and also there is a more significant presence of ICU members in the hospital HEC.
Relevant differences between public versus private-mixed hospitals.
| Public hospital | Private-mixed hospital | p | |
|---|---|---|---|
| ELC protocol | 28/54 51.9 per cent | 2/14 14.3 per cent | 0.015 |
| Tracheostomy ICD | 51/54 94.4 per cent | 11/14 78.6 per cent | 0.097 |
| Containment measures protocol available | 19/54 35.2 per cent | 8/14 57.1 per cent | 0.135 |
| Presence of an ICU member in the hospital HEC | 35/54 64.8 per cent | 12/14 85.7 per cent | 0.197 |
HEC: Healthcare Ethics Committee; ELC: end-of-life care; ICD: informed consent document; ICU: Intensive Care Unit.
The comparison between large-volume hospitals and small-volume hospitals (Table 3) shows a higher frequency of use of the briefing room to inform the family members, a higher presence of renal replacement therapy IDCs, and hemo-derivative transfusion IDCs in large-volume hospitals; also a higher frequency of use of end-of-life protocols and interventions by the patient's treating physician aimed at briefing the family, and a higher availability of LLSTs in small-volume hospitals.
Relevant differences between large-volume and small-volume hospitals (≥300 and <300 beds).
| Large-volume hospital (≥300 beds) | Small-volume hospital (<300 beds) | p | |
|---|---|---|---|
| ELC protocol | 4/18 22.22 per cent | 26/50 52 per cent | 0.029 |
| Specific briefing room | 17/18 94.4 per cent | 39/50 78 per cent | 0.16 |
| The treating physician and not the doctor on call is the informant | 14/18 77.8 per cent | 48/49 98 per cent | 0.016 |
| Extrarenal clearance techniques ICD | 7/18 38.9 per cent | 10/50 20 per cent | 0.113 |
| Hemo-derivative transfusion ICD | 17/18 94.4 per cent | 34/50 68 per cent | 0.084 |
| LLST document available | 6/18 33.3 per cent | 27/50 54 per cent | 0.132 |
ELC: end-of-life care; ICD: informed consent document; LLST: limitation of life-sustaining treatment.
The comparison between large-volume ICUs and small-volume ICUs (Table 4) shows a higher frequency of use of the briefing room, and a higher presence of renal replacement therapy IDCs, and hemo-derivative transfusion IDCs in large-volume ICUs; also a higher frequency of use of end-of-life protocols and interventions by the patient's treating physician aimed at briefing the family, a more significant presence of LLST documents, and a higher presence of ICU members in the hospital HEC in small-volume ICUs.
Comparison between large-volume (≥10 beds) and small-volume (<10 beds) Intensive Care Units.
| Large-volume ICU (≥10 beds) | Small-volume ICU (<10 beds) | p | |
|---|---|---|---|
| ELC protocol | 4/18 22.22 per cent | 26/50 52 per cent | 0.029 |
| Specific briefing room | 17/18 94.4 per cent | 39/50 78 per cent | 0.16 |
| The treating physician and not the doctor on call is the informant | 14/18 77.8 per cent | 48/49 98 per cent | 0.016 |
| Extrarenal clearance techniques ICD | 7/18 38.9 per cent | 10/50 20 per cent | 0.113 |
| Hemo-derivative transfusion ICD | 17/18 94.4 per cent | 34/50 68 per cent | 0.084 |
| LLST document available | 6/18 33.3 per cent | 27/50 54 per cent | 0.132 |
| There is or there was participation of an ICU physician in the hospital HEC | 10/18 55.5 per cent | 37/50 74 per cent | 0.146 |
HEC: Healthcare Ethics Committee; ELC: end-of-life care; ICD: informed consent document; LLST: limitation of life-sustaining treatment; ICU: Intensive Care Unit.
Table 5 shows the difference of ICUs with and without intensive care medicine residents. In ICUs with intensive care medicine residents, the presence of end-of-life care protocols, the fact that the patient's treating physician briefs the family, and the existence of LLST documents is more common. And the other way round, in ICUs without intensive care medicine residents, it is more common to have a briefing room to inform the family, renal replacement therapy IDCs, and hemo-derivative transfusion IDCs, and containment protocols.
Differences between Intensive Care Units with and without intensive care medicine residents.
| ICU with intensive care medicine MRs | ICU without intensive care medicine MRs | p | |
|---|---|---|---|
| ELC protocol | 25/42 59.5 per cent | 5/26 19.2 per cent | 0.001 |
| Specific briefing room | 32/42 76.2 per cent | 24/26 92.3 per cent | 0.090 |
| The treating physician and not the doctor on call is the informant | 40/41 97.6 per cent | 22/26 84.6 per cent | 0.070 |
| Extrarenal clearance techniques ICD | 8/42 19 per cent | 9/26 34.6 per cent | 0.15 |
| Hemo-derivative transfusion ICD | 27/42 64.3 per cent | 24/26 92.3 per cent | 0.019 |
| LLST document available | 25/42 59.5 per cent | 8/26 30.8 per cent | 0.021 |
| Containment measures protocol available | 14/42 33.3 per cent | 13/26 50 per cent | 0.172 |
ELC: end-of-life care; ICD: informed consent document; LLST: limitation of life-sustaining treatment; MR: medical resident; ICU: Intensive Care Unit.
Finally, Table 6 shows the differences between ICUs with and without actual presence of some of their doctors and/or nurses in the hospital HEC. It is more common than the treating physician briefs the family, that the information from the patient's clinical history is recorded properly, and that there are LLST documents available in ICUs that have representatives in the hospital HEC; also it is more common to have a briefing room to inform the families in ICUs without representatives in the hospital HEC.
Differences between Intensive Care Units with and without presence of actual representatives in the hospital healthcare ethics committees.
| Presence of ICU representatives in the hospital HEC | Lack of ICU representatives in the hospital HEC | p | |
|---|---|---|---|
| Specific briefing room | 35/47 74.5 per cent | 21/21 100 per cent | 0.013 |
| The treating physician and not the doctor on call is the informant | 45/47 95.7 per cent | 17/20 85 per cent | 0.153 |
| Records kept in the clinical history | 23/34 67.6 per cent | 7/15 46.7 per cent | 0.165 |
| LLST document available | 26/47 55.3 per cent | 7/21 33.3 per cent | 0.094 |
HEC: Healthcare Ethics Committee; LLST: limitation of life-sustaining treatment; ICU: Intensive Care Unit.
The 41/2002 Act regulates the autonomy of the patient and observes the patient's own rights and obligations on clinical information and documentation, and acts as the legislative framework under which indicators in bioethics operate.11 This act also regulates aspects of bioethical issues developed during the last few years included in our work such as the patient's anticipated will, informed consent, clinical history, and right to be informed on his/her state of health.
The assessment of quality indicators is essential to be able to identify any potential troubles and areas of improvement. In our setting, surveys are usually conducted in order to know the degree of compliance of these measures, or the presence of other related issues. Different surveys have been conducted during the last few years on the management of renal failure,12 the use of albumina,13 or the transfusion setting.14
When it comes to indicator #96, in our work we saw a smaller percentage, of nearly 56.4 per cent, of ICUs with end-of-life care protocols compare to the percentage by Estella et al.’s work4 where they assessed 39 ICUs for one whole week. This low implantation rate will probably be modified upwards after the implementation of an asystole donation protocol (Maastricht III) in more ICUs.15 The aspects of this survey relative to end-of-life issues and palliative care were already in the consensus document elaborated by the society task force on bioethics (TFB).16 In this document the focus is placed on fulfilling the goals of medicine in our patients, and when our patient's situation becomes irreversible, the focus should be placed on taking care of the patient and wait for a peaceful death. In prior studies, our society already advocated for end-of-life care protocols.17 Other English-speaking studies say the same thing.18,19 We have to say that end-of-life care protocols are more common in small-volume, public hospitals, and small-volume ICUs with intensive care medicine residents and representatives in the hospital HEC.
The information provided to the families (indicator #97) aims at briefing the patient on the very nature and ultimate purpose of the intervention that he/she is going to undergo.10 This information should be private and provided in a comfortable environment whenever possible. Nelson et al.’s work20 assesses issues related to end-of-life care within the ICU and claims that it is highly negative to have an inadequate place to meet the family. The data from our survey on this particular issue are acceptable (daily information provided by the treating physician inside a specific room for this purpose–the percentages were well above 80 per cent), though we should not forget that 18 per cent of ICUs claim not to have a specific room to inform the families. Large-volume hospitals, large-volume ICUs without intensive care medicine residents or representatives in the hospital HEC usually have briefing rooms like this. The treating physician is the one who usually briefs the patient in small-volume hospitals and small-volume ICUs with intensive care medicine residents and ICU members in the hospital HEC.
The lower presence of briefing rooms in ICUs with representatives in the hospital HEC who, theoretically speaking, are more sensitive toward bioethical issues, seems merely coincidental.
The documents with previous instructions/anticipated wills (indicator #98) summarize the desires expressed by patients on what kind of care they wish to receive whenever they cannot express themselves due to being incapacitated, and respect the patients’ autonomy, and anticipate the designation of a representative who will be the person in charge of the decision-making process.21 In our work we reviewed former documents with previous instructions in half the ICUs, and the subgroup analyses conducted do not show any significant differences in this intervention (same percentages regardless of the type of hospital and ICU, and the presence of absence of ICU members in the hospital HEC.)
Although providing the necessary information and obtaining an informed consent document (ICD) is a must before any medical procedures, the use of written ICDs is mandatory before certain procedures (indicator #99). They need to be filled out whenever patients give their authorization to a diagnostic or therapeutic procedure after being briefed by their doctor. The ICD collects information on the pros and the cons, the alternatives, and the consequences of any given healthcare process.22,23 The percentages of use of ICDs are low and insufficient in many common procedures such as extrarenal clearance techniques, or other common procedures in our daily practice such hemo-derivative transfusions. Although our working group showed its rejection to generic ICDs back in 2002,23 its use still seems inadequately high. In our series it is more common to see renal replacement therapy ICDs in large-volume hospitals and ICUs without intensive care medicine residents. In the subgroup analysis of the remaining ICDs we did not see any statistically significant clinical differences.
The LLST refers to the clinical decision made by the medical team, the patient, and the patient's family while keeping in mind the patient's own preferences to start or withdraw life support measures not deemed to be beneficial by the patient. It is justified on grounds of human dignity, respect for the patient;s autonomy and freedom of choice on his/her own death, and the medical team's responsibility is to observe this and not initiate any therapies that will not benefit the patient.2,3 Indicator #100 assesses the quality of the LLST by observing the prerequisites that need to be taken into account during the process, reporting it in the patient's clinical history, while contemplating the possibility that the ICU has forms available to itemize all particular aspects of the LLST. In our work, the presence of LLST documents is more common in small-volume hospitals and ICUs with intensive care medicine residents and representatives in the hospital HEC.
Measures aimed at physical containment should always be the last resort; these measures have to do with controlling all behaviors that put the patient at high risk, and they should be implemented whenever all verbal containment instructions, environmental measures, and drug containment measures have failed (indicator #101). These physical containment measures are indicated in the presence of clinical manifestations of significant agitation and, whenever possible, they should always be pre-noticed to the patient and to the patient's family and after the implementation of a specific protocol aimed at minimizing the risks and respecting the patient's physical integrity.24,25 Only 40 per cent of all ICUs included in our work have protocols for the implementation physical containment measures, and we did not find any differences in the subgroup analysis based on the type of hospital or ICU. The implementation of initiatives such as the HU-CI project (https://www.humanizandoloscuidadosintensivos.com) may result in the better management of patients and in a lower frequency of passive containment measures in ICU patients.
Our work fueled by the TFB has some limitations. In the first place, the survey, as any other survey, was not mandatory and had variable responses in different Spanish regions, which may lead to a certain distortion of the actual situation of our ICUs. To fight this information bias, several ways have been implemented to brief the ICUs on our latest developments. The lack of an ICU census in our country is an important limitation that goes against the necessary completeness of our survey and compromises its own representation. Secondly, there may be a certain veracity bias inherent to all surveys whenever we answer what we would like to see/have and not who we really are/what we really have. Thirdly, the ICUs were not selected at random and maybe the ICUs more interested in implementing significant advances in bioethics decided to respond to the survey, which may have made such survey look «too good». And in the fourth place, the initial contact with those in charge of bioethical issues at the ICUs may have some bias in the information gathered; however, the data collected in the survey such as the low frequency in the use of protocols for the management of LLST situations, and the lack of significant differences in the elements measures in the ICUs with and without members participating in the hospital HEC do not point in this direction. Eventually it shows a stationary image of the situation that may re-occur in the coming years.
This study has some really outstanding aspects too. In the first place, and as far as we know, it was the first study of these characteristics ever conducted in Spain and neighboring countries on these issues, since it deals with quality indicators of our own society; this uniqueness makes it impossible to compare our conclusions with those from similar studies. In the second place, the ICUs that responded to the survey can be found in almost all autonomous societies. In the third place, assessing quality indicators allowed us to detect areas of good practices in our ICUs, such as the daily disclosure of information to the families, the clinical decision-making processes agreed by the disciplinary team, and, ultimately, the presence of a briefing room. And in the fourth place, our results also show areas of future improvement, since less than 50 per cent of all ICUs have end-of-care protocols, LLST forms, or protocols for the implementation of containment measures. Also the low availability of ICDs for certain procedures such as non-emergent pacemakers, or vascular interventional procedures is an area that needs further improvement.
In sum, our study shows that the degree of implementation of quality indicators in bioethics in ICUs can be better. The indicator of information disclosure to the families is the one that is mostly followed, but improvement actions are needed on indicators associated with filling out the ICDs. The analysis of quality indicators as a management tool may help find areas that need further improvement in the ICUs.
Authors’ contributionAll authors have participated in the writing of this paper, and all have given their final approval to the paper.
Conflicts of interestsThe authors of this paper declare no conflicts of interest whatsoever.
Please cite this article as: López Camps V, García García MA, Martín Delgado MC, Añón Elizalde JM, Masnou Burrallo N, Rubio Sanchiz O, et al. Encuesta nacional sobre los indicadores de calidad en Bioética de la SEMICYUC, en los servicios de Medicina Intensiva en España. Med Intensiva. 2017;41:523–531.
The results of this work were presented at the LI SEMICYUC Congress (Valencia, Spain, June 19th–22nd, 2016) and at the 29th Annual Congress of the European Society of Intensive Care Medicine (Milan, Italy, October 1st–5th, 2016).