The clinical picture of SARS-CoV-2 infection (COVID-19) is characterized in its more severe form, by an acute respiratory failure which can worsen to pneumonia and acute respiratory distress syndrome (ARDS), and get complicated with thrombotic events and heart dysfunction. Therefore, admission to the Intensive Care Unit (ICU) is common.
Ultrasound, which has become an everyday tool in the ICU, can be very useful during COVID-19 pandemic, since it provides the clinician with information which can be interpreted and integrated within a global assessment during the physical examination. A description of some of the potential applications of ultrasound is depicted in this document, in order to supply the physicians taking care of these patients with a adapted guide to the intensive care setting.
Some of its applications since ICU admission include verification of the correct position of the endotracheal tube, contribution to safe cannulation of lines, and identification of complications and thrombotic events. Furthermore, pleural and lung ultrasound can be an alternative diagnostic test to assess the degree of involvement of the lung parenchyma by means of the evaluation of specific ultrasound patterns, identification of pleural effusions and barotrauma. Echocardiography provides information of heart involvement, detects cor pulmonale and shock states.
La infección por SARS-CoV-2 (COVID-19) se caracteriza por producir en las formas graves, un cuadro de insuficiencia respiratoria que puede evolucionar hacia neumonía y síndrome de distrés respiratorio agudo (SDRA), presentar complicaciones como fenómenos trombóticos y disfunción cardiaca, lo que motiva el ingreso en la Unidad de Cuidados Intensivos (UCI).
La ecografía, convertida en una herramienta de uso habitual en la UCI, puede ser muy útil durante la pandemia COVID-19 ya que la información obtenida por el clínico puede ser interpretada e integrada en la valoración global durante la exploración del paciente. Este documento describe algunas de sus aplicaciones con el objetivo de proporcionar una guía a los médicos responsables adaptado al paciente crítico con COVID-19.
Alguna de sus aplicaciones desde el ingreso en la UCI incluyen confirmar la correcta posición del tubo endotraqueal, facilitar la inserción segura de las vías, e identificar complicaciones y fenómenos trombóticos. Además, la ecografía pleuropulmonar puede ser una alternativa diagnóstica válida que permite evaluar el grado de afectación pulmonar, mediante el análisis de patrones ecográficos específicos, identificación del derrame pleural y del barotrauma. La ecocardiografía proporciona información acerca de la afectación cardíaca, detección del cor pulmonale y estados de shock.
The disease caused by SARS-CoV-2 (COVID-19) is characterized by pneumonia clinical presentation with fever and cough accompanied by multifocal nodular (round or oval) ground-glass opacities in the lungs that can progress to acute respiratory distress syndrome (ARDS) and requires admission to an Intensive Care Medicine Service (ICMS) in a high percentage of patients.1 On the other hand, infection due to SARS-CoV-2 can trigger thrombotic phenomena and severe deterioration of other organs like the heart.
Ultrasound can be a very useful tool during the management of the COVID-19 pandemic because it provides real-time non-invasive bedside images of patients admitted to intensive care units (ICU).2–4 Also, it increases safety in invasive procedures. In this context, the information obtained through different ultrasound imaging modalities can be interpreted and integrated in the patient’s complete general evaluation while being examined. Recently, several articles have been published on the role of ultrasound in the assessment of pulmonary and cardiovascular functions in patients with SARS-CoV-2.5–8
The Committee of Ultrasound Practice Accreditaion of the Spanish Society of Intensive and Critical Care Medicine and CoronaryUnits (SEMICYUC) has elaborated this document with some of the multiple applications of ultrasound as a guide for doctors who treat patients with COVID-19, especially during ICU admission (Table 1).
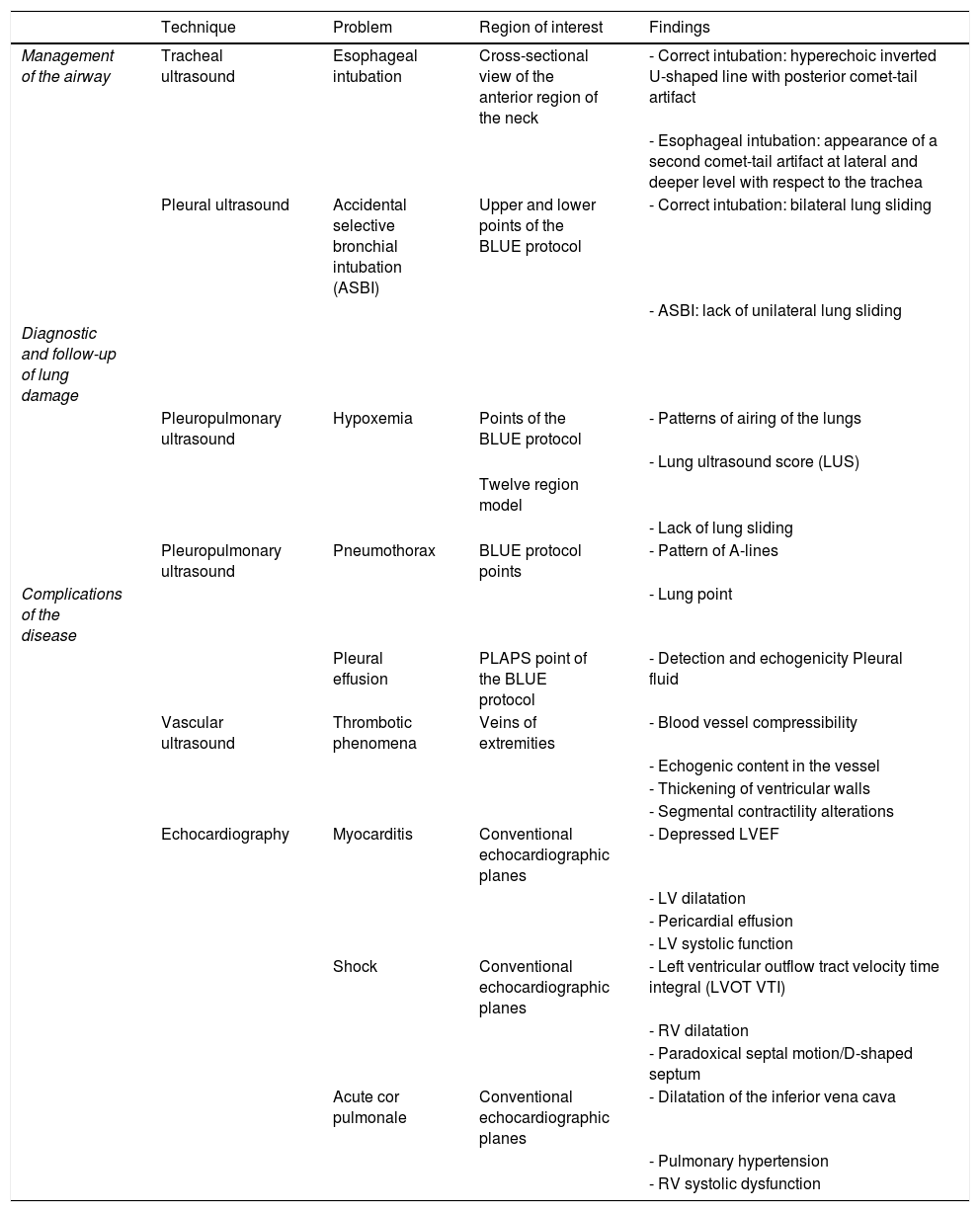
Main applications of the ultrasound during the management of the current COVID-19 pandemic in the Intensive Care Medicine Service (ICMS) setting.
| Technique | Problem | Region of interest | Findings | |
|---|---|---|---|---|
| Management of the airway | Tracheal ultrasound | Esophageal intubation | Cross-sectional view of the anterior region of the neck | - Correct intubation: hyperechoic inverted U-shaped line with posterior comet-tail artifact |
| - Esophageal intubation: appearance of a second comet-tail artifact at lateral and deeper level with respect to the trachea | ||||
| Pleural ultrasound | Accidental selective bronchial intubation (ASBI) | Upper and lower points of the BLUE protocol | - Correct intubation: bilateral lung sliding | |
| - ASBI: lack of unilateral lung sliding | ||||
| Diagnostic and follow-up of lung damage | ||||
| Pleuropulmonary ultrasound | Hypoxemia | Points of the BLUE protocol | - Patterns of airing of the lungs | |
| - Lung ultrasound score (LUS) | ||||
| Twelve region model | ||||
| - Lack of lung sliding | ||||
| Pleuropulmonary ultrasound | Pneumothorax | BLUE protocol points | - Pattern of A-lines | |
| Complications of the disease | - Lung point | |||
| Pleural effusion | PLAPS point of the BLUE protocol | - Detection and echogenicity Pleural fluid | ||
| Vascular ultrasound | Thrombotic phenomena | Veins of extremities | - Blood vessel compressibility | |
| - Echogenic content in the vessel | ||||
| - Thickening of ventricular walls | ||||
| - Segmental contractility alterations | ||||
| Echocardiography | Myocarditis | Conventional echocardiographic planes | - Depressed LVEF | |
| - LV dilatation | ||||
| - Pericardial effusion | ||||
| - LV systolic function | ||||
| Shock | Conventional echocardiographic planes | - Left ventricular outflow tract velocity time integral (LVOT VTI) | ||
| - RV dilatation | ||||
| - Paradoxical septal motion/D-shaped septum | ||||
| Acute cor pulmonale | Conventional echocardiographic planes | - Dilatation of the inferior vena cava | ||
| - Pulmonary hypertension | ||||
| - RV systolic dysfunction |
BLUE, bedside lung ultrasound in emergency; LV, left ventricle; LVEF, left ventricular ejection fraction; PLAPS point: posterolateral alveolar and pleural syndrome; RV, right ventricle.
The arrival of portable ultrasound machines has made them available for use in most ICMSs. Also, the existence of wireless ultrasound transducers or probes connected to a tablet or smartphone connected through USB, although of lower image quality compared to bigger machines, allows us to obtain ultrasound images from patients with COVID-19. They bring more advantages compared to portable consoles like smaller sizes, lighter weight, they are easy to carry, power up fast, have a certain degree of asepsis, and can be easily covered with 1-use only disposable plastic cases. All of it reduces the risk of contamination and nosocomial infection, while facilitates cleaning and sterilization.7,9
The use of dedicated portable ultrasound transducers specifically for patients with COVID-19 is advised with special precautions of asepsis and sterility to reduce cross infections. Covering the keyboard and the probe is advised too.10
The ultrasound machine should be cleaned following the recommendations established to fight the current pandemic11,12 (Appendix B annex 1 of the digital supplementary data [DSD]).
Ultrasound airway managementThe airway management through orotracheal intubation (OTI) and while on mechanical ventilation (MV) is the usual procedure for the management of patients with severe acute respiratory failure (ARF) due to SARS-CoV-2. OTI is a technique that can release aerosols, which is why it should be performed by a properly trained professional through videolaryngoscopy preferably and with the recommended personal protection equipment (PPE).13
Confirming the correct positioning of the orotracheal tube (OTT) after the OTI is essential. It is often based on the capnography and physical examination (direct visualization of the passage of the OTT through the vocal cords, symmetric elevation of both hemothoraces, lack of noise at epigastral level, auscultation of vesicular murmur, and condensation of the tube with ventilation).14–16 The suboptimal access to resources like capnography and the inconvenience associated with the PPE when performing a complete physical examination make the ultrasound a very useful imaging modality in this context.
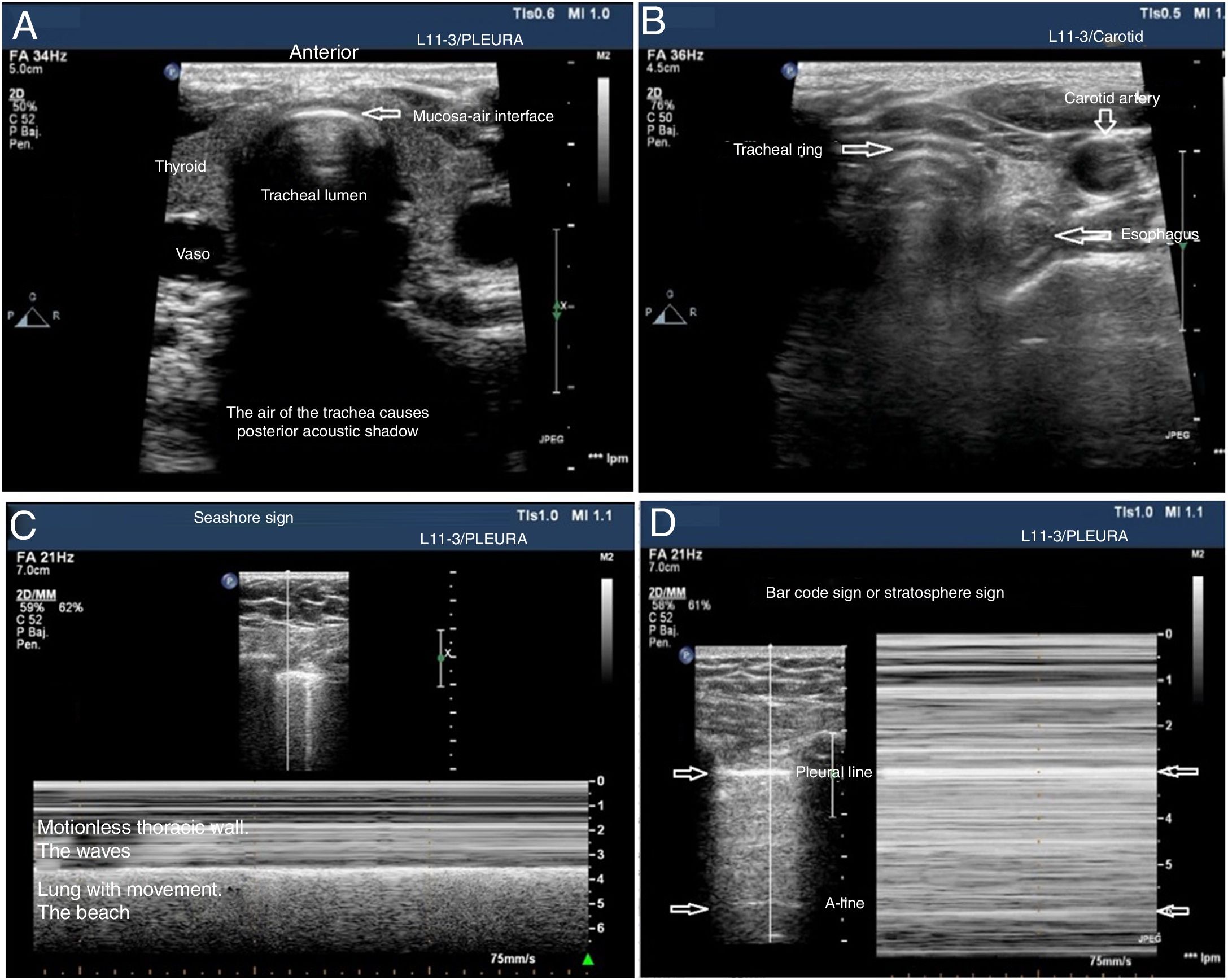
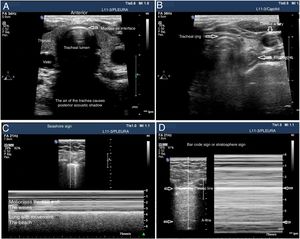
The tracheal ultrasound has proven to be non-inferior to capnography to rule out esophageal intubation.17 For this purpose, it is necessary to use a linear or convex transducer and acquire the cross-sectional view from the anterior region of the neck, in the mid-line above the sternal notch. In this plane, the trachea looks like a hyperechogenic inverted U-shaped line generated by the mucosa-air interface and a posterior comet-tail artifact (Fig. 1A). The correct intubation of the trachea does not cause any changes in the image described since no new interface between the patient’s airway and the OTT is generated (both contain air). Also, because the OTT is located behind the artifact generated by the trachea (which would limit its visualization). On the echocardiography, the accidental intubation of the esophagus generates a new interface between the esophageal mucosa and the air inside the OTT giving rise to a second comet-tail artifact that originates at the deepest level of the trachea and is lateral to it (Fig. 1B). The main advantage of this type of study is that ventilation does not need to be started to detect the incorrect position of the OTT, thus avoiding the possibility of aspiration of gastric content; its limitations are the impossibility to see the OTT directly from inside the airway and the patient’s possible anatomical alterations.
Utility of the tracheal and pleural ultrasound for OTI verification purposes. A) Tracheal ultrasound image showing the trachea with its posterior acoustic shadow surrounded by the thyroid. B) Tracheal ultrasound image with the transducer displaced towards the neck left region. The arrow points at the esophagous. C) Pleural ultrasound image with normal airing pattern indicative that the lung is insuflated. D) Pleural ultrasound image with bar code sign indicative that the lung is not insuflated. Possible contralateral selective intubation.
Pleural ultrasonography provides direct anatomical information on the expansion of the lungs and entry of air into the lungs.18 It can replace auscultation and chest x-rays as the method to rule out accidental selective bronchial intubation (ASBI). For this purpose, it is required to use a linear or convex probe to examine, at least, the upper and lower points of the BLUE protocol in both hemithoraces.19 In these planes, on the 2D ultrasound, the pleura appears as a horizontal hyperechogenic line with movement generated by the slide of the parietal pleural surface over the visceral pleura with respiratory movements called lung sliding or lung displacement sign.20 On M-mode it can also be registered statically by cutting the pleural line producing an image called seashore sign. The presence of both signs in each hemithorax is indicative of bipulmonary insufflation (Fig. 1C). The consequence of ASBI is the lack of airing of the contralateral lung that can be found by the absence of lung sliding and a typical «bar code» pattern or stratosphere sign on M-mode (Fig. 1D). The transformation of a unilateral bar code pattern into a normal lung sliding pattern associated with the partial removal of the OTT is indicative that the initial position of the tube corresponds to an ASBI of contralateral lung (Fig. 7). The main advantages of this kind of examination are the possibility of showing the airing of the lung without having to use the stethoscope and its immediacy compared to the chest x-ray. The main limitation is the existence of differential diagnoses of the stratosphere sign (lack of ventilation, pneumothorax, tumors, etc.).
Pleural and pulmonary ultrasoundIn the COVID-19 pandemic, the main trait of critically ill patients is pneumonia progressing towards ARDS with a characteristic diagnostic pattern on the CT scan.1
Assessing patients with ARF using the BLUE protocol (bedside lung ultrasound in emergency) is one of the well-known and consolidated applications of pulmonary ultrasound.19 Although the medical literature available on the use of pulmonary ultrasound (PU) to assess patients with suspected SARS-CoV-2 infection and its potential applications is still scarce, some data that show the good correlation between PU findings and thoracic CT scans.6
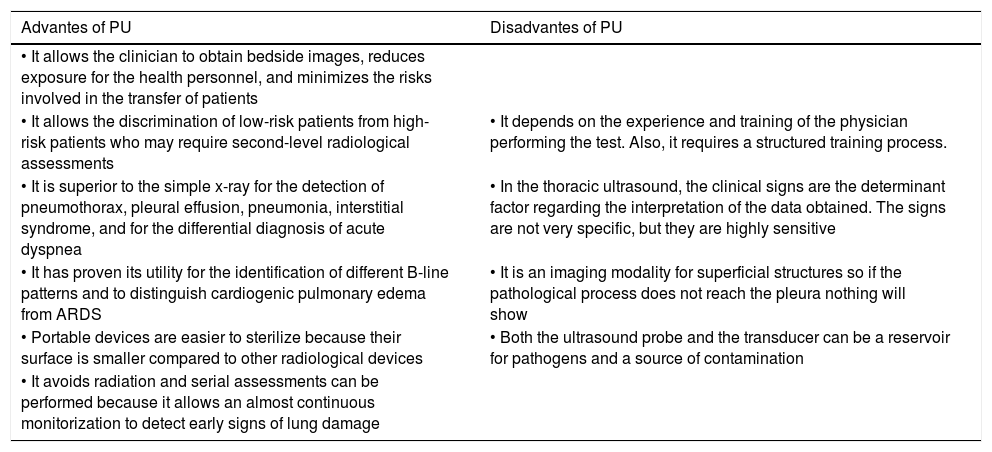
Although the CT scan is the recommended imaging modality, the difficulties associated with moving the patient, the high risk of spreading the microorganism during the transfer, and the later disinfection of the radiology rooms make PU a valid diagnostic imaging modality for the bedside assessment of the degree of pulmonary damage through the analysis of specific ultrasound patterns.21 The PU should not be used separately but complement the echocardiography in the assessment of several conditions of the critically ill patient.22,23 The advantages and limitations of PU are shown on Table 2.
Main advantages and disadvantages of pulmonary ultrasound.
| Advantes of PU | Disadvantes of PU |
|---|---|
| • It allows the clinician to obtain bedside images, reduces exposure for the health personnel, and minimizes the risks involved in the transfer of patients | |
| • It allows the discrimination of low-risk patients from high-risk patients who may require second-level radiological assessments | • It depends on the experience and training of the physician performing the test. Also, it requires a structured training process. |
| • It is superior to the simple x-ray for the detection of pneumothorax, pleural effusion, pneumonia, interstitial syndrome, and for the differential diagnosis of acute dyspnea | • In the thoracic ultrasound, the clinical signs are the determinant factor regarding the interpretation of the data obtained. The signs are not very specific, but they are highly sensitive |
| • It has proven its utility for the identification of different B-line patterns and to distinguish cardiogenic pulmonary edema from ARDS | • It is an imaging modality for superficial structures so if the pathological process does not reach the pleura nothing will show |
| • Portable devices are easier to sterilize because their surface is smaller compared to other radiological devices | • Both the ultrasound probe and the transducer can be a reservoir for pathogens and a source of contamination |
| • It avoids radiation and serial assessments can be performed because it allows an almost continuous monitorization to detect early signs of lung damage |
PU, pulmonary ultrasound.
The examination is performed in the supine decubitus position by dividing each hemothorax in quadrants.24 The articles that study the utility of assessing different ultrasound patterns of airing of the lungs to obtain a score analyze 6 areas in each hemithorax outlined by 3 longitudinal lines at sternal level (long axis of the clavicle towards the diaphragm), anterior and posterior axillary as anatomical references outlining 3 different areas: anterior (1 and 2), lateral (3 and 4), and posterior (5 and 6). By drawing a cross-sectional line at nipple level these areas are divided into upper and lower regions. This is how 6 different examination regions are established so they can be studied through an evolutionary analysis. Actually, a recent pilot study has proven that it is non-inferior to the protocols that use more quadrants.25 Pleural effusion (PE) and the presence of consolidation are analyzed on region 6 or equivalent to the PLAPS point (posterolateral alveolar and/or pleural syndrome) of the BLUE protocol24 (Appendix B annex 3 of DSD).
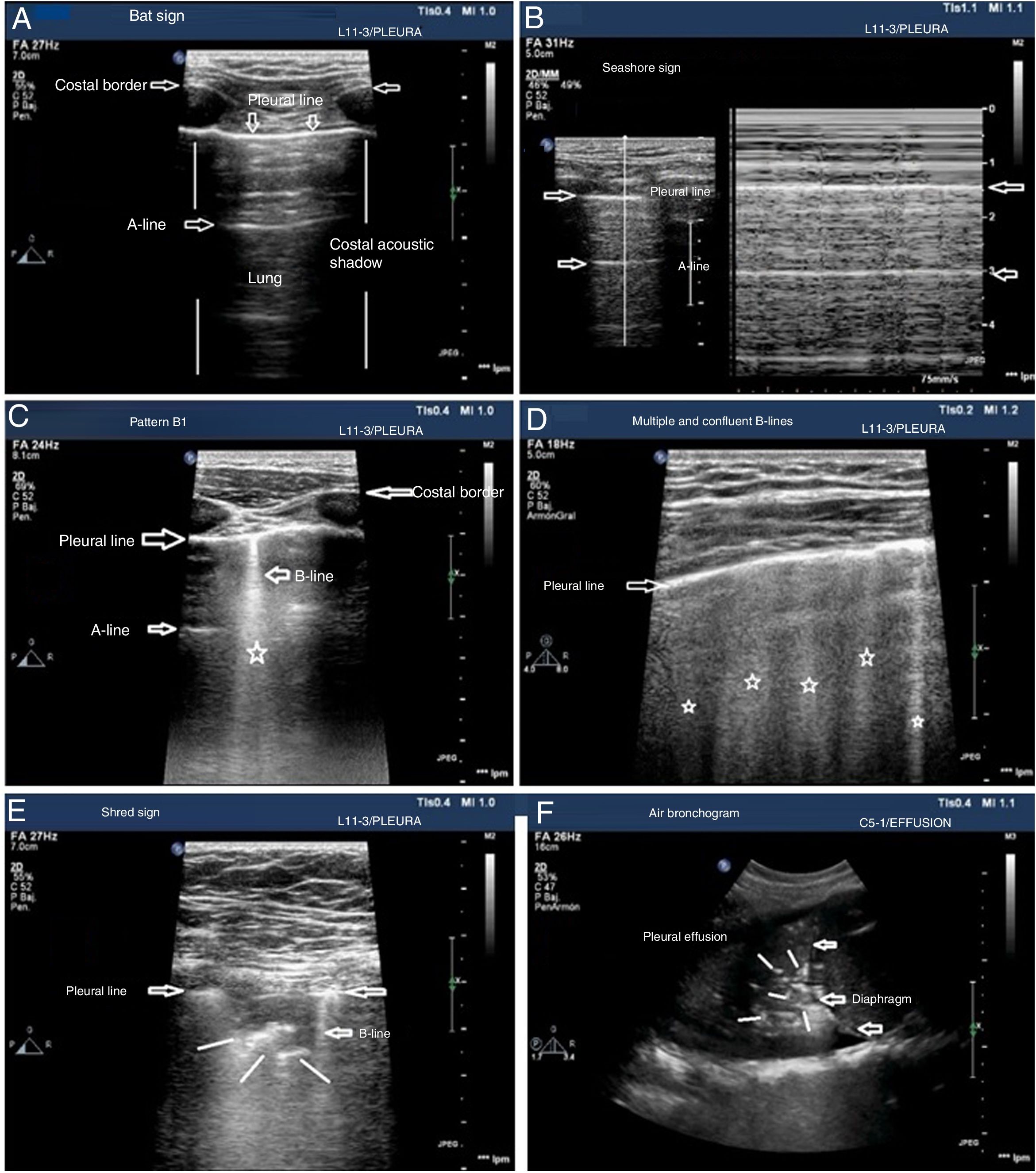
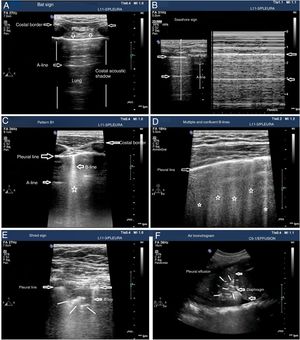
The patterns of airing of each pulmonar region are (Fig. 2):
- •
Pattern A: typical pattern of normal airing. Pleural line with preserved sliding, presence of well spaced A-lines and ≤ 2 B-lines (Fig. 2A and figure B).
- •
Pattern B1: presence of more than 2 well spaced, diffuse B-lines in different thoracic regions called septal rockets (Fig. 2C).
- •
Pattern B2: confluent B-lines separated between them by ≤ 3mm (ground-glass rockets) due to a more severe loss of aired lung (Fig. 2D).
- •
Pattern C: lung consolidation suggestive of significant loss of airing of the lung due to the accumulation of fluid and/or cells in the alveoli. The consolidation can be found anywhere in the hemithorax but applying the transducer to the PLAS point detects 95% of the cases (Fig. 2E and figure F).
Patterns of airing of the pulmonary region. A) Typical pattern of normal airing (profile A in the BLUE protocol) showing 2D imaging. B) Pattern A on M-mode. C. Pattern B1. D) Pattern B2. E) Pulmonary consolidation (profile C in the BLUE protocol) showing shred sign. F) Air bronchogram. A, B, C, D, and E: linear probe study; F, convex probe.
Patterns A, B1, and B2 include the presence of lung sliding (Appendix B annex 3 of the DSD).
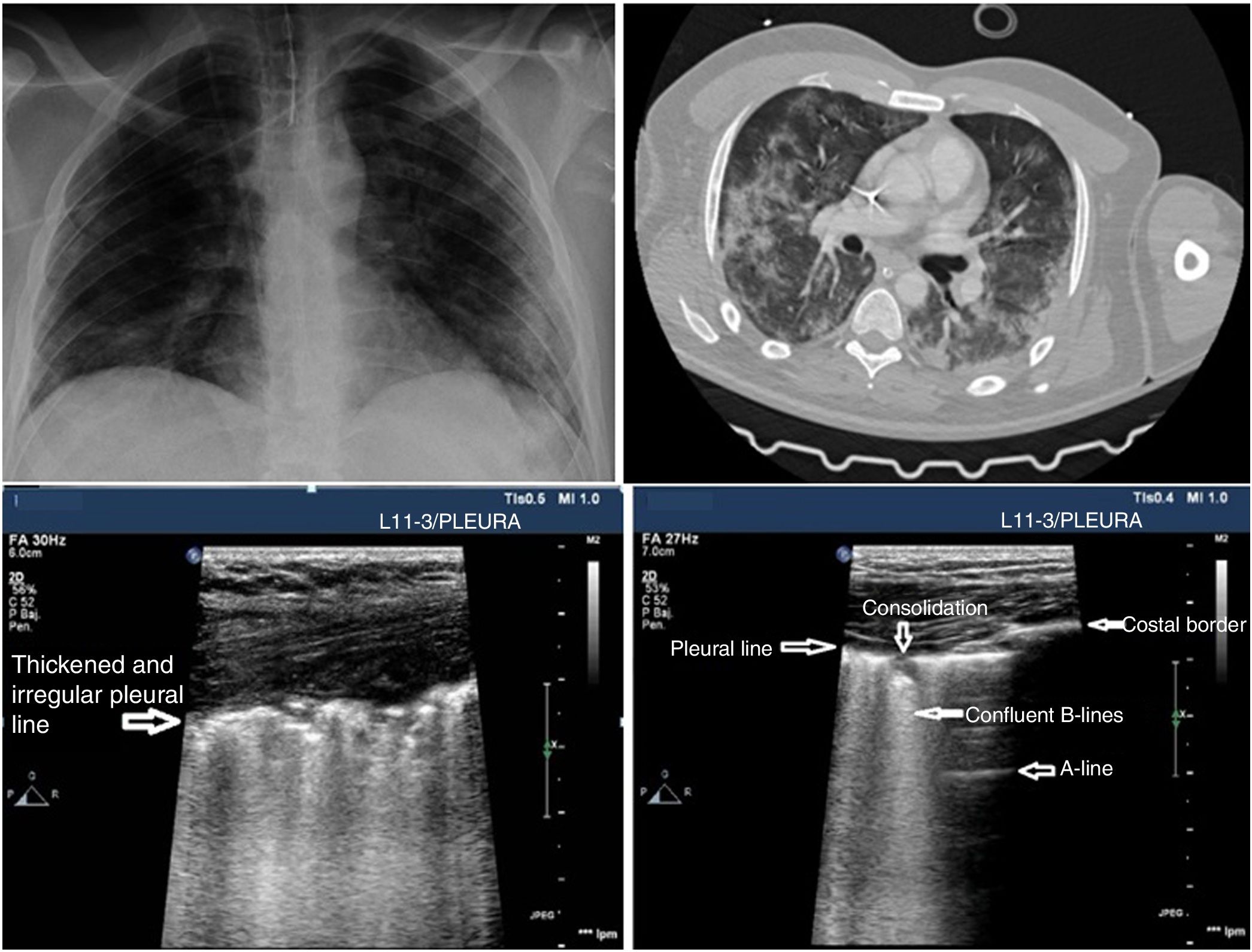
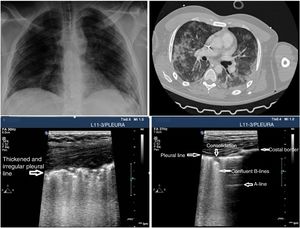
In the case of patients with COVID-19 the typical findings are based on disease progression. The first signs on the PU show an irregular distribution of focal B-lines, later confluent, that spread towards several regions of the pulmonary surface. Disease progression is represented by the appearance of small subpleural consolidations with a thickened and irregular pleural line and areas associated with white lung, regions with pattern A patches, and anomalous areas without massive PE. Disease progression will show a consolidation pattern, especially in dependent regions of the body with or without air bronchogram and its increasing spread across the pulmonary surface, indicative of progression to ARF that may require MV.
The air/fluid correlation determines the ultrasound appearance of the lung and provides different patterns based on the degree of aired parenchyma, which will allow us to assign a different score to each region under study.
PU can be useful to monitor the progression of the disease using the Lung Ultrasound Score (LUS).26,27 This scoring system uses a model to explore 12 regions (Appendix B annex 4 of the DSD). The score is based on the worst ultrasound pattern registered in each of the 12 regions studied by assigning the following values: pattern A=0 points; pattern B1=1 point; pattern B2=2 points; pattern C=3 points. The overall score is the sum of the points assigned to each region (from 0 that would be indicative of a totally aired lung to a maximum of 36, that would be describing a totally condensed lung). During the natural progression of infection due to SARS-CoV-2 at the ICU setting, the patients’ infiltrates often improve on the chest x-ray. However, the PU will still show persistent pathological findings with pleural line thickening and abundant B-lines that may be predictive of a difficult MV disconnection or weaning failure27 (Fig. 3). The LUS score tells us about the aired pulmonary mass and provides clinical and prognostic information. That is why the LUS can be used to predict what patients may need to be admitted to the ER after presenting with fever and dyspnea. Also, to detect patients in whom MV weaning may fail while at the ICU. Further studies are needed to validate this scoring system in patients with pneumonia due to SARS-CoV-2.
Other pulmonary conditions, although rare in the early stages of pneumonia due to SARS-CoV-2 can appear during the natural progression of the disease such as pneumothorax or pleural effusion.
PneumothoraxDetecting pneumothoraces is important to assess MV- or iatrogenic-induced barotrauma after recruitment maneuvers of central venous cannulation (CVC). The PU has been confirmed as an alternative imaging modality to the chest x-ray in the diagnosis of pneumothorax with a 98% specificity and a 75% sensitivity.28,29
Although a systematic study can be conducted in unstable patients in the supine decubitus position and since air tends to occupy the thorax anterior side, the anterior regions should be examined first. A linear or micro-convex transducer is enough to assess the pleural line and the lung artefacts (Fig. 4).
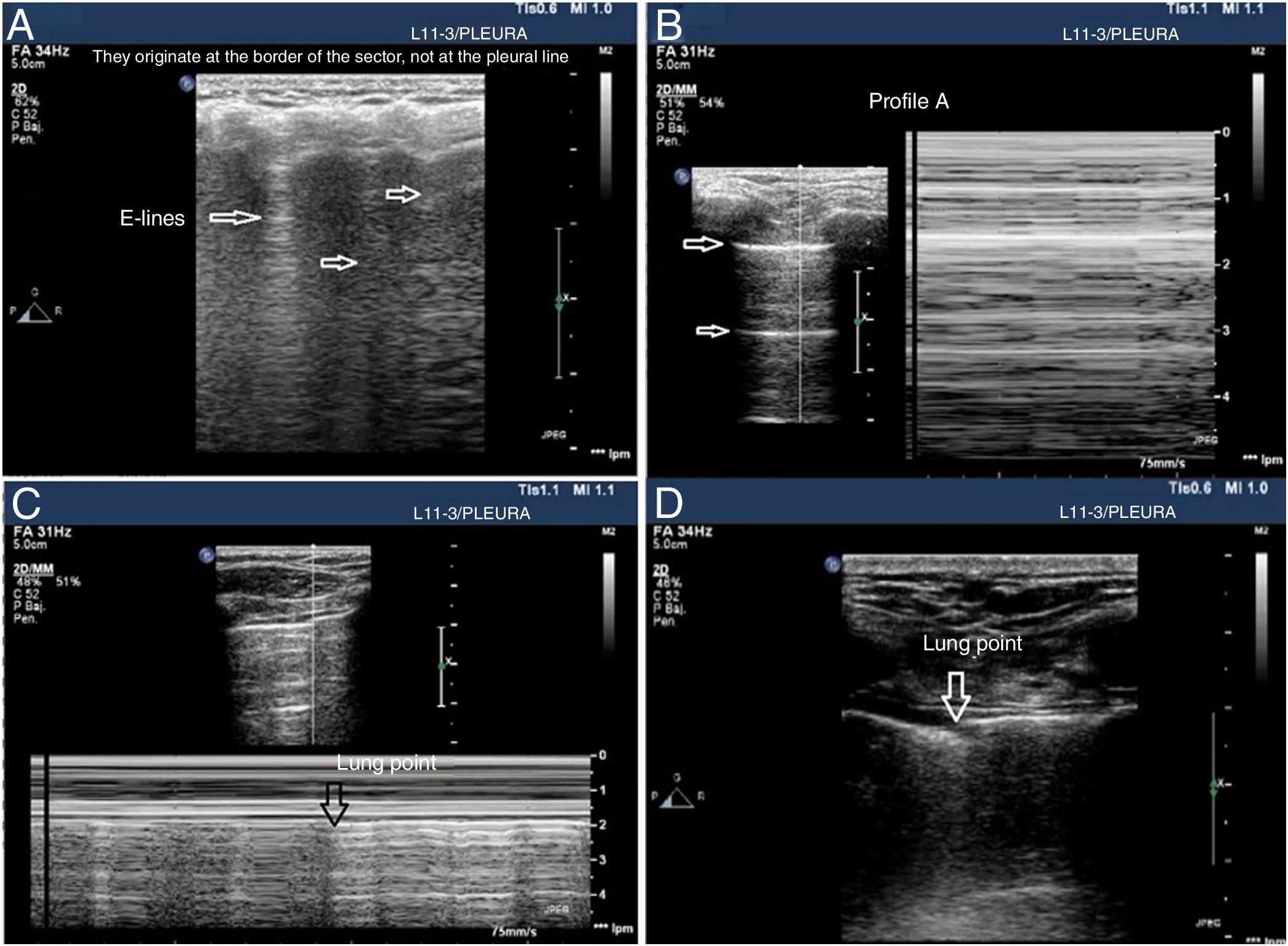
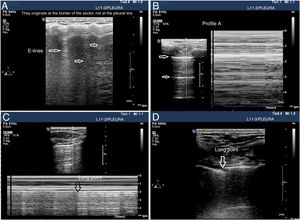
Signs of barotrauma on the pleural ultrasound. A) E-lines, vertical artifact not originated at the pleural line in relation with subcutaneous emphysema. B) A-line pattern and lack of pleural sliding as confirmed with the M-mode, on the left side, representative of the strathosphere sign. C) Image confirming the presence of pneumothorax and the separation point of the visceral from the parietal pleura (lung point) on M-mode. D) 2D image of the lung point and point of separation of both pleuras.
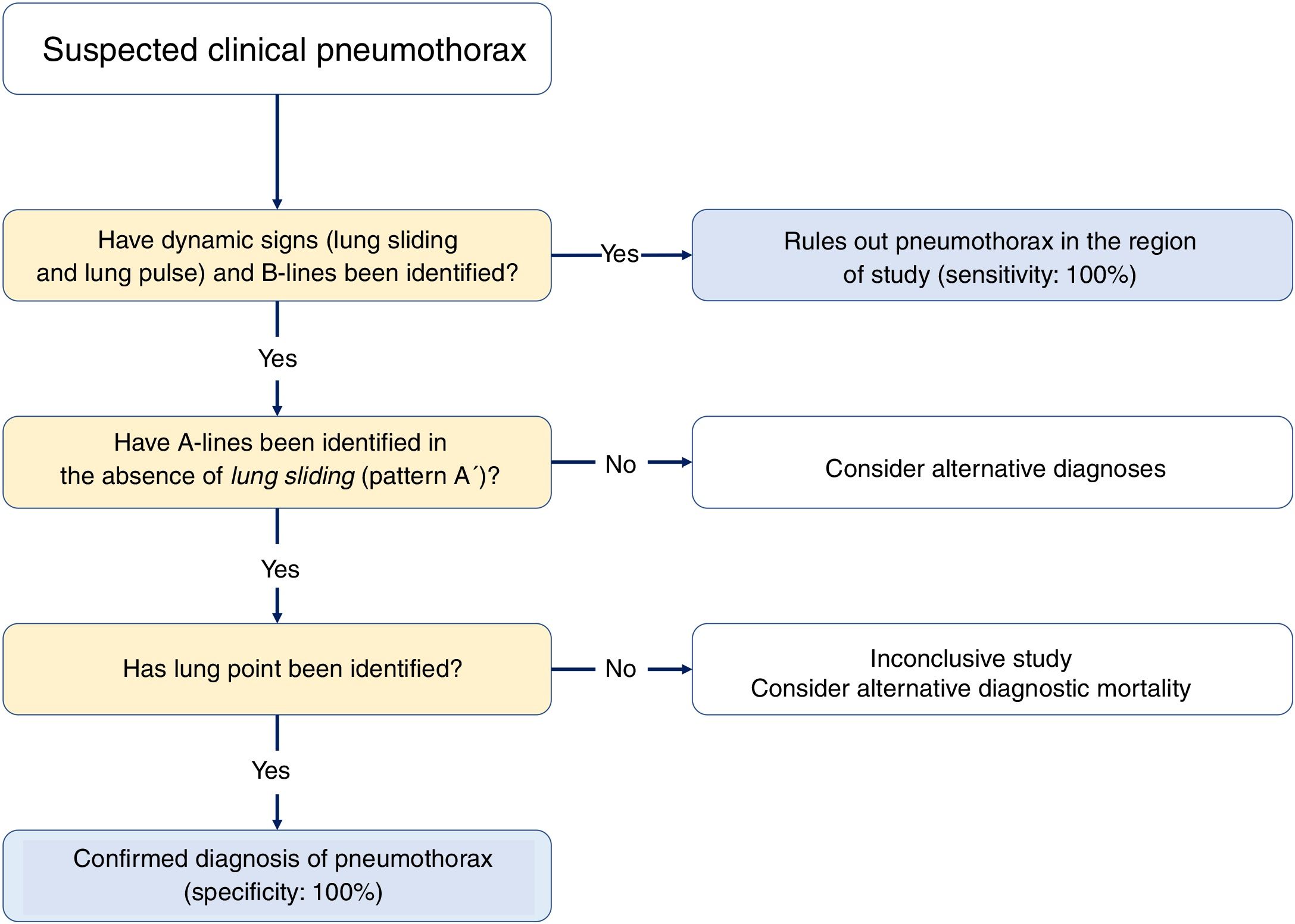
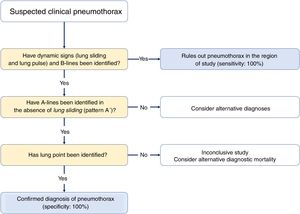
Three signs rule out the presence of pneumothorax20,30:
- •
The presence of lung sliding is indicative that both pleural layers are in contact and rules out the presence of pneumothorax (NPV, 100%) in the space where the transducer is placed. However, its absence is not confirmed by its low specificity.
- •
Seeing 1 B-line only is indicative that both layers are attached, which rules out the presence of pneumothorax with a 100% NPV. Extra care should be paid here to distinguish B-lines from E-lines (vertical hyperechoic lines originated at the thoracic wall soft tissues in the presence of subcutaneous emphysema) (Fig. 4A).
- •
Presence of lung pulse: the rhythmic movement of the pleural line in synchrony with the heart beats in the absence of lung sliding is indicative that both the parietal and visceral pleuras are in contact. Nonetheless, regional ventilation is impaired (eg, atelectasis) because the air between both pleural layers is blocking its transmission.
Sequential diagnosis is achieved with 2 signs (Fig. 8).
- •
Lung sliding abolished with the presence of A-lines (profile A’ in the BLUE protocol) (Fig. 4B).
- •
Find the lung point between the collapsed lung and the pneumothorax collection of air (lung point) (Fig. 4C and D). It is a dynamic sign indicative of an impaired normal and abolished sliding during 2D ventilation or by the succession of normal images (seashore sign) during inspiration and horizontal lines (strathosphere sign or bar code sign) durante exhalation on M-mode. It is a specific sign with a 66% sensitivity and a 100% specificity to diagnose pneumothorax.30 The more lateral and lower the lung point is to the thoracic wall, the more it has spread. A very posterior or absent lung point is indicative of a massive pneumothorax with complete lung atelectasis.31
Pleural effusion (PE) is a rare complication of COVID-19. We should not forget that the International Sonsensus Conference on Lung Ultrasound establishes that «for the detection PE, the UP is more accurate than the x-ray in the supine position and almost as accurate as the CT scan» (level A).32
PE is often gravity dependent and accumulates in dependent regions of the thorax. That is why examination should start close to the diaphragm with a convex probe being the PLAPS point the location where it is better detected. PE appears as an often anechoic, echo-free space between the parietal and visceral layers of the pleura and always above the diaphragm.33 The latter allows us to distinguish PE from ascites.
The appearance of PE should be interpreted within the clinical context. Its appearance should not be assessed separately in the therapeutic decision-making process since an anechoic PE can be indicative of both an exudate and a transudate34 (Appendix B annex 5 of the DSD).
Vascular ultrasoundOne of the most common findings in severe disease due to SARS-CoV-2 is a profoundly impaired hemostatic function whose occurrence has prognostic implications. Over two thirds of the patients who die of this disease meet the diagnostic criteria of disseminated intravascular coagulation (DIC) proposed by the ISTH (International Society on Thrombosis and Haemostasis). However, its incidence rate among survivros is < 1%.35
The predominant component of an impaired hemostatic function is not bleeding diathesis but prothrombotic state associated with venous thromboembolic phenomena and microvascular thrombosis with high levels of d-dimer and fibrinogen. In critically ill patients, these phenomena are associated with a higher rate of thrombosis in intravascular devices and vascular occlusive phenomena.35,36
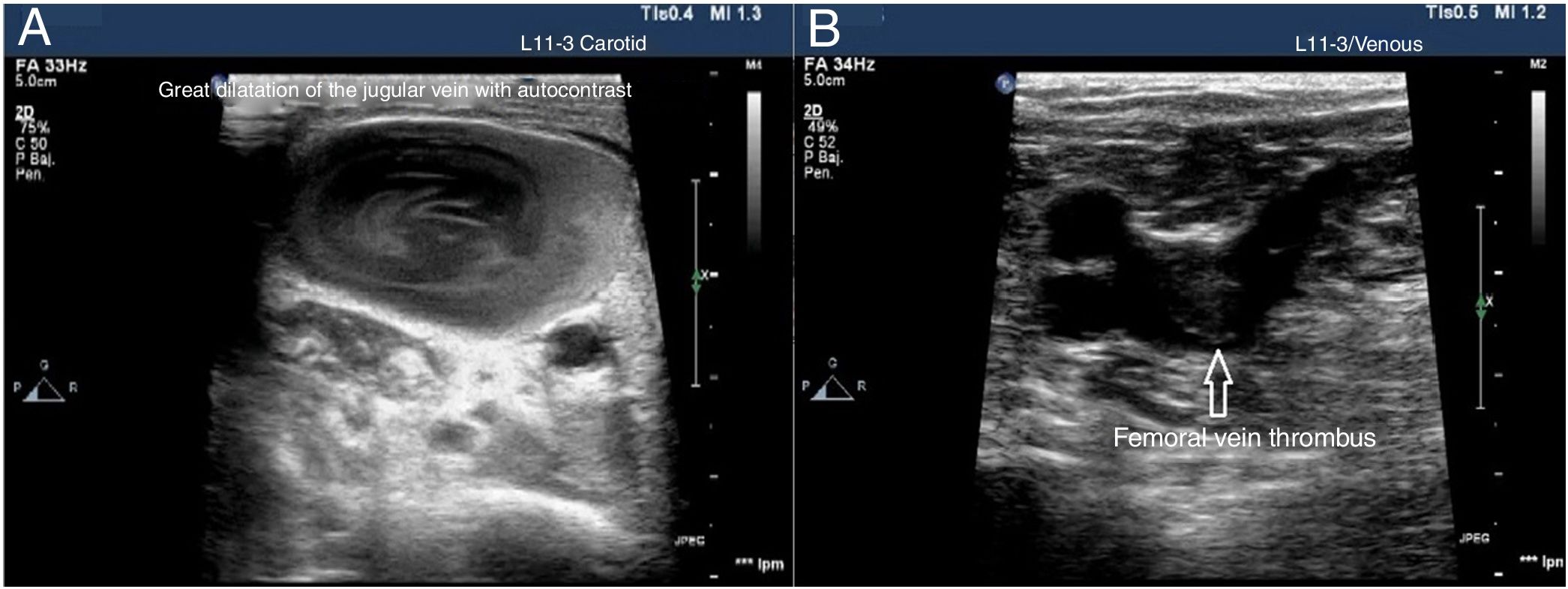
Vascular ultrasound identifies certain thrombotic phenomena and allows us to start a therapeutic strategy for control and prevention, which eventually may improve prognosis.37 For its study it is necessary to use a high-frequency linear probe (7−15MHz) to explore the venous territory with special attention to CVC insertion areas and complete the study of the venous blood vessels of both legs all the way up to the popliteal vein. It has been estimated that up to 90% of all embolisms occurred in patients with pulmonary thromboembolisms come from the lower extremity proximal veins. Thrombi appear as echogenic material inside the blood vessel. However, immature thrombi may not be echogenic and there are times when they are partially compressible due to their gelatinous consitency. Therefore, the main diagnostic criterion is the lack of vessel compressibility and not the visualization of a blood clot.
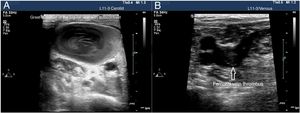
The use of vascular ultrasound increases safety and effectiveness during CVC insertion compared to techniques based on anatomical references.38–40 Before starting the puncture, vascular accesses should be explored to determine which is the most suitable one and detect vascular anatomical variables.41 In cases of venous thrombosis and/or anatomical impairment, the access vein should be changed42 (Fig. 5). The size of the blood vessel that is going to be catheterized is important and, in ideal conditions, the external diameter of the catheter should correspond, at most, to a third of the internal diameter of the vein (for example, a 4-Fr catheter requires a 4 mm-wide vein). The risk of thrombosis increases when the caliber of the catheter is wider than recommended.43 It is necessary to keep the measures of asepsis including the use of sterile gel and a protective cover for the probe to reduce the risk of catheter related bacteremia.
Two approaches are advised here: ultrasound-guided, the ultrasound is performed prior to the puncture to study the target vessel, analyze its size, depth, patency, and vascular puncture using the Seldinger technique. This technique visualizes the tip of the needle while inside the blood vessel during the procedure and the advance of the metal guidewire and later ultrasound-guided placement of the catheter: after confirming the size of the blood vessel and lack of inner thrombi, the puncture is performed without seeing the needle with the ultrasound transducer39 (Appendix B annex 6 of the DSD).
EchocardiographyCardiovascular and cardiac damage due to SARS-CoV-2 seems to be associated with a higher mortality rate. Myocardial lesion is confirmed through higher biomarker levels of myocardial damage (troponin I or T or natriuretic peptides) that has been reported with higher rates (from 7% to 28%),44–48 and is associated with more ICMS admissions, greater need for MV, coagulopathy, acute kidney injury, and a higher mortality rate.44–47
The mechanism of myocardial damage is a complex one, but the direct lesion mediated by the angiotensin-converting enzyme 2 (ACE 2), hypoxia, and the possibility of a cytokine inflammatory response have been postulated in its pathogenesis.49,50 There are 2 different types of myocardial lesions:
- •
Subacute: it consists of the progressive increase of the biomarker levels of myocardial damage and inflammation (D-dimer, ferritin, lactate, etc.) with slow progression and gradual impairment of organ functions probably associated with a lesion due to cytokine storm or hemophagocytic lymphohistiocytosis that eventually leads to the death of the patient 2/3 weeks after symptom onset.
- •
Acute: damage of sudden onset with poor progression that causes sudden myocardial dysfunction within a few hours. It is consistent with myocarditis or stress cardiomyopathy. With the actual evidence base available this second pattern is apparently rare.51–53
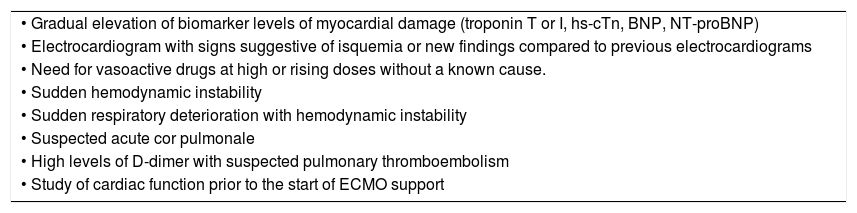
The echocardiography, both basic and advanced, is essential for the management of patients with SARS-CoV-2 infection with shock or myocardial damage and for the detection of acute cor pulmonale (ACP) (Table 3).
Indications of echocardiography in patients with COVID-19.
| • Gradual elevation of biomarker levels of myocardial damage (troponin T or I, hs-cTn, BNP, NT-proBNP) |
| • Electrocardiogram with signs suggestive of isquemia or new findings compared to previous electrocardiograms |
| • Need for vasoactive drugs at high or rising doses without a known cause. |
| • Sudden hemodynamic instability |
| • Sudden respiratory deterioration with hemodynamic instability |
| • Suspected acute cor pulmonale |
| • High levels of D-dimer with suspected pulmonary thromboembolism |
| • Study of cardiac function prior to the start of ECMO support |
ECMO, extracorporeal membrane oxygenation.
It is essential to know the interactions between the lung and the heart of patients on MV as well as the interactions between these patients and vasoactive and inotropic drugs and systems of extracorporeal membrane oxygenation (ECMO).54,55
Use of echocardiography for the management of acute cardiac lesionsEchocardiographic studies should be conducted according to the international recommendations on heart quantification.56 In light of the high volume of patients due to the current pandemic it is important to minimize the time devoted to each study and perform targeted echocardiographies to answer easy questions.57
In patients with high biomarker levels of myocardial damage, the differential diagnosis of acute myocardial infarction (AMI), myocarditis or pulmonary thromboembolism (PTE) should be performed.
Left ventricular systolic function (LVSF) is the most studied element in echocardiographies and is an essential aspect of echocardiographic studies. Keeping a proper cardiac output not only depends on cardiac contractility, but also on preload, afterload, heart rate, and a proper segmental contraction synchrony. The effect of all of these factors should be taken into consideration when interpreting echocardiographic findings.
Left ventricular ejection fraction (LVEF) is often estimated using the biplane method. However, qualitative or semiquantitative visual assessments have proven a good correlation with the standard method in critically ill patients due to the worse definition of endocardial border in this population, which is why it can be a valid alternative.2,58 We should mention that LVEF is mostly influenced by left ventricular (LV) contractility and afterload.59 Therefore, it does not adequately show the contractile capabilities of the left ventricle in an acute situation, but the way the cavity adapts itself to loading conditions, which can have very important dynamic changes in states of shock.60
The disease due to SARS-CoV-2 often causes damage to segmental contractility. When these alterations are identified in the theoretical territory of a coronary artery the possibility of acute ischemia associated with a type-1 AMI should be suspected, especially if it is associated with consistent electrocardiographic changes and high biomarker levels of myocardial damage. However, studies published during the pandemic show a higher rate of type-2 AMIs. When these alterations are diffuse or not consistent with the territory of theoretical distribution of a coronary artery, alternative diagnoses like myocarditis or stress cardiomyopathy should be suspected especially in unstable patients.44,50,61
Myocarditis is an entity often associated with virus infections and has a wide constellation of clinical presentations that go from mild clinical signs to cardiogenic shocks or ventricular arrhytmias that may eventually lead to the death of the patient. The SARS-CoV-2 coronavirus is capable of damaging the myocardium through direct or indirect action mediated by the inflammatory response it triggers. This clinical status should be suspected in patients with electrocardiographic alterations and high levels of cardiac biomarkers. Although the gold standard for diagnosis purposes is endomyocardial biopsy, in the routine clinical practice it is established based on the cardiac magnetic resonance (CMR) imaging findings. However, given the potential risks involved in the transfer of critically ill patients to undergo an MRI for diagnostic purposes and the technical difficulties of MV, the echocardiography is a not a very reliable imaging modality in many patients. It can help diagnose fulminant myocarditis after visualization of the acute thickening of ventricular walls due to interstitial edema and segmental contractility alterations that trigger a reduced LVEF and, occasionally, left ventricular dilatation, right ventricular (RV) dysfunction, and pericardial effusion without signs of hemodynamic compromise.57
Stress cardiomyopathy, apical ballooning syndrome or the Tako-Tsubo syndrome is a clinical syndrome often triggered by a stressor (such as SARS-CoV-2 infection). It is characterized by transient left ventricular dysfunction due to segmental contractility regional alterations associated with electrocardiographic changes and high levels of cardiac biomarkers.62 Up to 4 different patterns of segmental alterations have been reported. However, the most common of all is hypokinesia or akinesia of medial and apical segments with compensatory hypercontractility of basal segments that give the left ventricle a typical balloon appearance. The differential diagnosis of the least common patterns with acute coronary syndrome or acute myocarditis requires performing a cardiac magnetic resonance (CMR) imaging.63
Use of echocardiography in patients with shockAlthough the echocardiography does not provide continuous information on the patient’s hemodynamic status, it is an excellent tool to characterize the state of shock, choose the best therapeutic option, and assess the response to it.4 The European Guidelines on Hemodynamic Monitoring in Critically ill Patients64 recommend performing an echocardiography as the best way to study cardiac function. Also, they establish the quantification of the left ventricular outflow tract velocity time integral (LVOT VTI) as the only parameter to estimate the systolic volume of these patients by omitting the estimate of the LVOT area and monitoring its changes after therapeutic measures like the administration of fluids, inotropic, and vasopressor drugs60,64 (Appendix B annex 7 of the DSD).
In the case of the overall left ventricular systolic function measusing the LVOT VTI and the qualitative visual assessment of LVEF is advised. Other parameters more difficult to obtain, time-consuming or associated with greater limitations are ill-advised.60
In patients on ECMO support, it is necessary to perform an echocardiography to assess the cardiac function prior to cannulation in order to choose the most suitable modality: veno-venous or veno-arterial.65–67
Use of echocardiography for the detection and management of acute cor pulmonaleThe acute cor pulmonale (ACP) is defined as a right ventricular failure due to primary pulmonary disorder that triggers a sudden increase of pulmonary vascular resistances.54 The leading causes of ACP in patients admitted to an ICMS are ARDS and PTE.68 The echocardiographic findings that define ACP are69:
- •
Right ventricular dilatation
- •
Paradoxical septal motion
- •
Dilatation of the inferior vena cava (IVC)
- •
Pulmonary hypertension
- •
Right ventricular systolic dysfunction
The ACP is a common cause of circulatory failure in ARDS and can be associated with a higher mortality rate in its more severe forms.70 The detection of ACP leads to the use of ventilatory strategies «with protective cardiopulmonary parameters» that should control the main determinants of an increased right ventricular afterload: proper control of plateau pressures (< 27 cmH2O), and distension (< 18 cmH2O), optimization of oxygenation, and correction of hypercapnia.71,72
Assessing the right ventricular sizeThe assessment of the right ventricle should start in the parasternal long-axis view (PLAX) of the left ventricle that shows the size of the RV and its relation to the LV. The parasternal short-axis view (PSAX) at papillary muscle or mitral valve level shows the RV hugging the LV in a crescent shape. When the RV is dilated, the anterolateral wall is difficult to see and there is clockwise rotation of the cavity. The apical 4-chamber (A4C) view provides the best information on the size of the RV because we can compare it to the LV. But for this to happen the A4C view needs to be focused on the RV to make sure there is no foreshortening phenomenon of the RV and the left ventricular outflow tract cannot be seen (Appendix B annex 8 of the DSD).73 Although standard measures of the RV can be taken with specific cuts, the correlation between both ventricles is often used to establish the right ventricular dilatation69:
- •
RV/LV<0.6 → normal size of the RV.
- •
RV/LV=0.6–1 → mild dilatation of the RV.
- •
RV/LV>1 → severe dilatation of the RV.
Interventricular septum (IVS) is a structure that both ventricles share although functionally it is part of the LV in normal conditions. The IVS is often convex towards the RV and concave towards the LV and keeps this morphology during the entire cardiac cycle. However, in the ACP, the IVS is flattened during the entire cardiac cycle giving it its characteristic d-shape.
Assessment of the inferior vena cava (IFC)The IFC is studied in the subcostal plane. In the ACP we may find a dilated IFC (> 21mm) with scarce respiratory collapse. Its size and collapsibility are the ultrasound method used to assess pressure in the right atrium.
Assessment of pulmonary hypertensionThe assessment of pulmonary hypertension using the echocardiography is performed by estimating pulmonary artery systolic pressure (PASP) using the tricuspid regurgitation pressure gradient (Appendix B annex 9 of the DSD). When the acoustic window is limited or suboptimal, a better signal can be acquired through the IV administration of 10mL of agitated saline solution. The DAP can be measured through a CVC or estimated from the size and respirophasic variation of the IFC. In acute situations of pressure overload the RV cannot adapt fast enough and the pressures found in these cases are often relatively low (< 60mmHg).
When the PASP cannot be estimated using the method already described, the pulmonary artery acceleration time (PAAT) can be used (Appendix B annex 9 of the DSD).
Assessing the right ventricular systolic functionIn order to quantify the right ventricular systolic function, the recommendations established by the international guidelines should be followed.56,74 The parameters most commonly used are the tricuspid annular plane systolic excursion (TAPSE) and the tricuspid annular peak systolic velocity (TAPSV) mediated by the Tissue Doppler imaging (ST wave). These parameters are easy to obtain, reproducible, and have a good correlation with other more complex methods to study the right ventricular systolic function54 (Appendix B annex 10 of the DSD).
Use of echocardiography in patients in the prone decubitus positionA large number of patients with COVID-19 develop ARDS and need MV in the decubitus pone position to treat ARF over long periods of time. During this time, many patients require an echocardiographic study for different reasons. However, placing patients in the supine decubitus position just to undergo this test can worsen their respiratory failure. The alternatives are using a transesophageal transducer or performing a transthoracic echocardiography (TTE) in the prone decubitus position. To that end, the patient needs to be placed in the swimmer position with his left upper extremity above his head. The patient’s left shoulder needs to be elevated on a pillow and the transducer placed at 5th left intercostal space level in the midclavicular line to obtain all the measures associated with the apical plane.75 (Fig. 6).
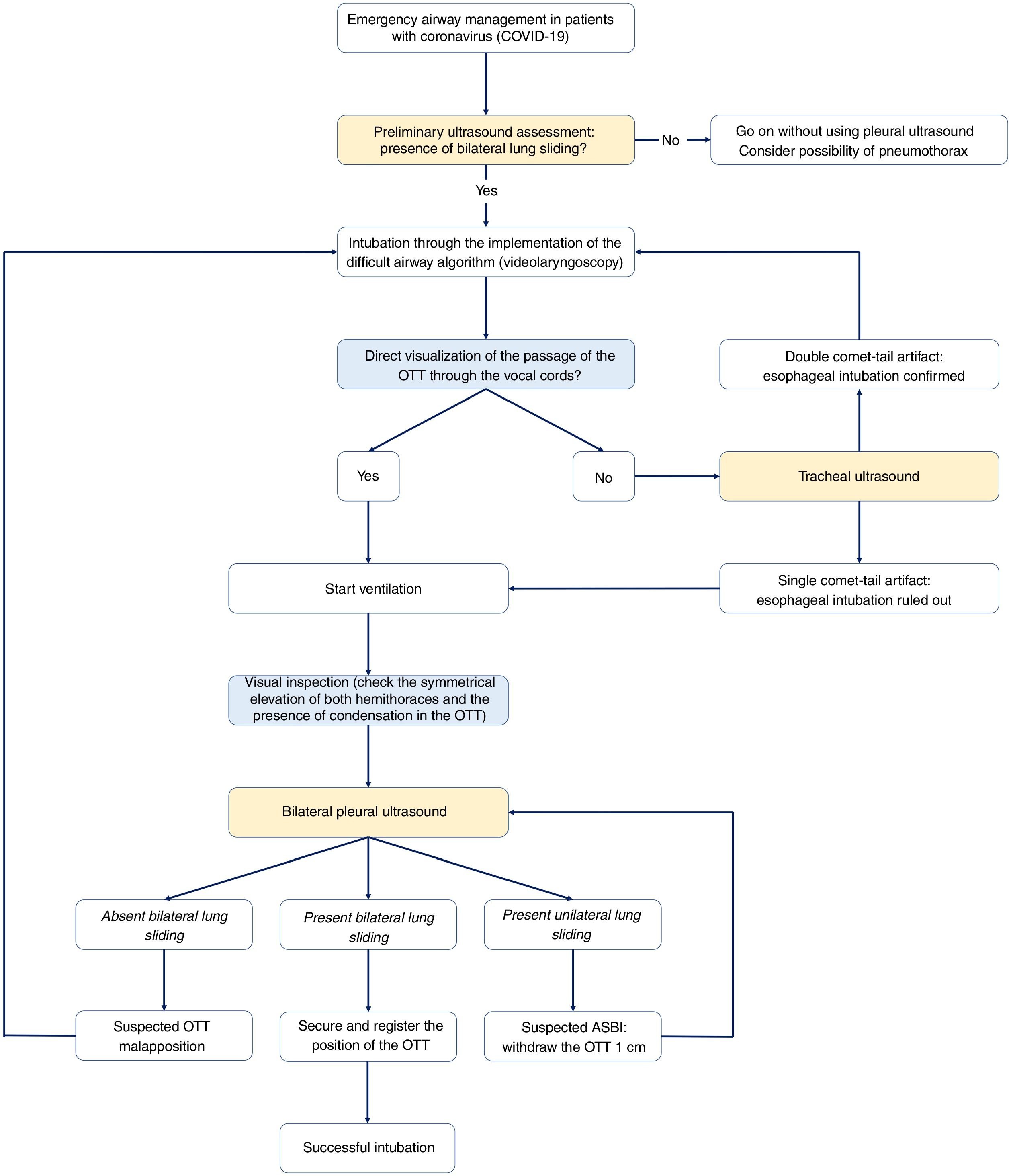
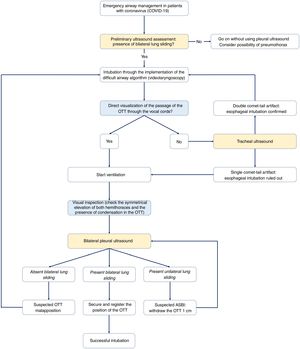
Emergency airway management in patients with coronavirus.
Algorithm for emergency airway management in patietns with coronavirus though comprehensive management including visual inspection and ultrasound use. Before starting intubation, it will be necessary to confirm the presence of bilateral lung sliding. After preliminary verification, the OTI will be initiated through the implementation of the difficult airway management algorithm of every center. Here the videolaryngoscopy as the first device is advised. The direct visualization of the passage of the OTT through the vocal cords facilitates the start of ventilation, the identification of visual signs of a proper intubation, and the adjustment of the position of the OTT using the bilateral pleural ultrasound. Being unable to see the passage of the OTT through the vocal cords rules out the possibility of esophageal intubation before starting ventilation to avoid the pulmonary aspiration of gastric content.
None reported.
Please cite this article as: Fraile Gutiérrez V, Ayuela Azcárate JM, Pérez Torres D, Zapata L, Rodríguez Yakushev AL, Ochagavía Calvo A. Revisión narrativa de la ecografía en el manejo del paciente crítico con infección por SARS-CoV-2(COVID-19): aplicaciones clínicas en medicina intensiva. Med Intensiva. 2020;155:551–565.