To determine the accuracy and usefulness of noninvasive continuous hemoglobin (Hb) monitoring in critically ill patients at risk of bleeding.
DesignAn observational prospective study was made, comparing core laboratory Hb measurement (LabHb) as the gold standard versus transcutaneous hemoglobin monitoring (SpHb).
SettingPediatric Intensive Care Unit of a tertiary University Hospital.
PatientsPatients weighing >3kg at risk of bleeding.
InterventionsSpHb was measured using the Radical7 pulse co-oximeter (Masimo Corp., Irvine, CA, USA) each time a blood sample was drawn for core laboratory analysis (Siemens ADVIA 2120i).
VariablesSociodemographic characteristics, perfusion index (PI), pleth variability index, heart rate, SaO2, rectal temperature, low signal quality and other events that can interfere with measurement.
ResultsA total of 284 measurements were made (80 patients). Mean LabHb was 11.7±2.05g/dl. Mean SpHb was 12.32±2g/dl (Pearson 0.72, R2 0.52). The intra-class correlation coefficient was 0.69 (95%CI 0.55–0.78) (p<0.001). Bland–Altman analysis showed a mean difference of 0.07±1.46g/dl. A lower PI and higher temperature independently increased the risk of low signal quality (OR 0.531 [95% CI 0.32–0.88] and 0.529 [95% CI 0.33–0.85], respectively).
ConclusionsSpHb shows a good overall correlation to LabHb, though with wide limits of agreement. Its main advantage is continuous monitoring of patients at risk of bleeding. The reliability of the method is limited in cases with poor peripheral perfusion.
Determinar la validez y precisión de un método de medición continua transcutánea de la concentración de hemoglobina (Hb) en pacientes críticos con riesgo de sangrado.
DiseñoEstudio observacional prospectivo comparando el estándar de referencia con la determinación transcutánea de hemoglobina (SpHb).
ÁmbitoUnidad de cuidados intensivos pediátricos de un hospital universitario de tercer nivel.
PacientesMuestra consecutiva de pacientes con peso >3kg y riesgo de sangrado.
IntervencionesMedición de SpHb mediante el cooxímetro de pulso Radical7 (Masimo Corp., Irvine, CA) en cada extracción sanguínea con determinación de Hb analizada con estándar de referencia (Siemens ADVIA 2120i).
VariablesVariables epidemiológicas, índice de perfusión (IP), índice de variabilidad pletismográfica, frecuencia cardiaca, SaO2, temperatura rectal, baja calidad de señal, así como otros factores que pueden afectar a la medición.
ResultadosSe realizaron 284 mediciones (80 pacientes). La media de Hb por el analizador central fue de 11,7±2,05g/dl. La media de SpHb fue de 12,32±2g/dl (Pearson 0,72, R2 0,52). El índice de correlación intraclase fue de 0,69 (IC95%: 0,55-0,78), p<0,001. El diagrama de Bland-Altman mostró una diferencia media entre ambos métodos de 0,66±1,46g/dl. Un menor IP y una mayor temperatura rectal incrementaron de forma independiente el riesgo de baja calidad de la señal (OR 0,531 [IC 95%: 0,32-0,88] y 0,529 [IC 95%: 0,33-0,85], respectivamente).
ConclusionesLa SpHb presenta buena correlación con la obtenida por el analizador central, aunque los límites de concordancia son amplios. Su principal ventaja es la posibilidad de monitorización continua en pacientes con riesgo de sangrado. La fiabilidad de este método es limitada en casos de mala perfusión periférica.
Anemia is a common complication in pediatric intensive care units (PICU) and amounts to up to 74% of hospitalized patients.1 In critical patients anemia is the consequence of blood losses due to acute traumatic hemorrhages in the postoperative period, especially due to cardiac or trauma surgeries, repeated blood extractions, chronic disorders, iron and other deficiency disorders, liver dysfunction or disseminated intravascular clotting, among others. In one multicentre, observational, prospective study conducted in children admitted at pediatric intensive care units, Bateman et al.1, found that 96% of these patients had their blood test analyzed, and 74% exhibited daily blood losses. Patients with anemia had longer hospital stays and spent more days on mechanical ventilation. Forty-one percent of children with anemia developed it during their hospital stay while only 33% had anemia at the moment of hospital admission. Conducting repeated blood test analyses can cause iatrogenic anemia that is present in up to 90% of patients during the third day of admission in adult intensive care units.2,3 This fact becomes more important in younger patients since the blood volume needed for extraction is not related to the child's weight, which means that larger volumes will be associated with the patient's blood volume.4 Thus, the more serious the patient's condition is, the more blood losses due to blood extractions will occur.3
However, the diagnosis of anemia cannot be clinically validated and requires confirmation through blood sample analysis. The setback of trying to determine the blood hemoglobin concentration using conventional methods is that obtaining lab results can delay therapy.
The capacity to rapidly and accurately determine a patient's blood hemoglobin concentration may be useful and critical in various clinical settings, especially in intensive medicine, which has led to the development of point-of-care (POC) devices, that is, devices available at the patient's bedside so that there is no need to bring the blood sample to the core lab. Nevertheless, the accuracy of these methods has been questioned in some studies.5–9
These are the non-invasive modalities available today: pulse Co-oximetry (Pulse CO-Oximetry™; Masimo Corp., Irvine, CA, USA), occlusion spectroscopy (OrSense™, Ness Ziona, Israel) and transcutaneous reflectance spectroscopy (Haemospect, MBR Optical Systems, Herdecke, Germany).10
The technology behind pulse co-oximetry is based on pulse oximetry and provides a continuous non-invasive monitoring of the blood hemoglobin concentration. This method allows us to distinguish between the different characteristics of light absorption and the different subtypes of hemoglobin, and through the implementation of certain algorithms is capable of determining the total blood hemoglobin concentration.11 Theoretically speaking, one device capable of continuously measuring the blood hemoglobin concentration would have results earlier and alert clinicians on any drops in all those cases of hidden hemorrhages–all this added to the advantages of non-invasive measurements.
There are some studies conducted in the pediatric population, most of them in the surgical setting and with hemodynamically stable patients.12–14
This is why we decided to conduct one study aimed at determining the validity and accuracy of this monitoring method in relation to the determination achieved at the core lab in PICU-hospitalized patients at risk of bleeding.
Material and methodsGoalDetermine the validity and accuracy of one continuous transcutaneous method for measuring the blood hemoglobin (Hb) concentration in critical patients at risk of bleeding.
DesignProspective observational design.
Field of studyPICU of a third-level referral academic hospital.
Study populationThe study included patients admitted at the PICU with risk of bleeding (postoperative cardiac or trauma surgery patients, polytraumatized patients and patients with serious coagulopathy in the context of systemic inflammatory syndrome response), weighting >3kg for a period of twelve (12) months.
Study protocolWhen each blood sample was extracted, the concentration of Hb at the core lab (Siemens ADVIA 2120i) was compared to the concentration of Hb shown by the Radical/7.8.0.1 pulse co-oximeter (Masimo Corp., Irvine, CA). Several epidemiological variables were recorded (genre, age in months, weight, race), reason for hospital admission together with the following parameters: perfusion index (PI), plethysmographic variability index (PVI), heart rate (HR), SaO2, rectal temperature (RT), the Richmond Agitation Sedation Scale (RASS), low signal quality detected by the monitor, and all those situations that might affect measurement (Vasoactive-Inotropic Score [VIS score], mechanical ventilation).
The PI is defined as the coefficient of pulse blood flow in relation to the non-pulsed or static flow in peripheral tissue and is one non-invasive way to measure peripheral perfusion. The PVI measures dynamic changes occurring in the PI during the respiratory cycle, which are expressed in percentages and calculated by the device following this formula: [(maximum PI−minimum PI)×100]/maximum PI.
Based on the manufacturer's specifications, the finger of the hand (ringer finger, middle finger, and index finger of the non-dominant hand) and the hallux of the foot were the locations used, while limbs with recent lesions, surgeries or vascular catheters–mainly arterial catheters were avoided. The sphygmomanometer was always placed in a limb different to where the sensor had been placed. After its placement the explored area was covered with an opaque bag supplied by the manufacturer in order to avoid pollution and light interference. If there was no stable measurement after two (2) attempts another measurement was attempted in a different location before recording the case as non-detectable. Likewise, the low signal quality alarm was recorded, which according to the manufacturer's specifications, goes off in situations of excessive environmental light pollution, shunt between photo emitter and photo detector due to sensor misplacement, patients making moves, or between the placement site and the sensor.
The R1 20L sensor was used in patients whose weight was between 3 and 10kg. For individuals over 10kg, the R2 20 sensor was used.
Statistical analysisIn the descriptive analysis, data are expressed as mean and standard deviation for variables of normal distribution, and as mean and interquartile range (IQR) for variables that do not meet the conditions of normality. The categorical variables are described as absolute frequency and percentage.
The Pearson correlation coefficient was obtained as well as the interclass correlation coefficient (ICC) that was calculated using a two-factor alpha model, random effects and absolute consensus with a 95% confidence interval and the Bland–Altman diagram in order to compare the hemoglobin concentration established by the pulse co-oximeter (SpHb) and the hemoglobin concentration established by the core lab analyser (tHb). Tolerance limits were established in differences of ±1g/dl. In order to calculate the difference between the two (2) methods, the absolute difference was used in the first place–including both negative and positive values. In the Bland–Altman analysis, positive and negative numbers were used to know these differences.
Using the multivariate linear regression analysis, the influence exerted by the independent variables gathered on the absolute difference between both methods was analyzed. Using the multivariate logistic regression analysis, the correlation between low signal quality (yes/no) and different potential variables associated was studied. A 5% statistical-significance level was taken into account. For the selection of the final multivariate model in both cases, a backward step-wise regression proceeding (Wald test statistics) was used initially including all the variables that might have something to do with the dependent variables and the variables that exhibited statistical significance in the bivariate analysis. The Akaike information criterion (AIC) was used to confirm how good the adjustment of the model really was.
For the statistical analysis, the IBM® SPSS® Statistics 20.0 software for Macintosh® was used. The Bland–Altman diagram was built using the R software–version 3.2.2.
Ethical aspectsThis paper has been approved by the Regional Research Ethics Committee. Participation in this study was voluntary. The patients’ legal representatives were verbally informed and given one copy of the written informed consent. The study authors declare that they have followed the good clinical practice and principles contained in the Declaration of Helsinki.
Results284 measurements were taken in 80 patients averaging 14.5 months of age (range 3–60 months) and 8.5kg of weight (range 4.35–18.5kg). In 15 determinations (5.2%), the SpHb could not be recorded due to poor peripheral hyperperfusion uptake, and in 36 determinations (12.7%) there was poor signal quality as the Radical7 station exhibited.
The SaO2 was 98% (IQR 96–100%), the HR 123±24lpm, the RT 36±2°C, the PI 1.5 (IQR 0.93–3.32) and the PVI 17 (IQR 11–27.25).
Most patients included were postoperative cardiac surgery patients (76.4%), and polytraumatized patients (14.8%), while 8.8% had been admitted to the hospital PICU for other reasons such as post-adenoidectomy bleeding (4), upper GI bleeding (2), or septic shock (1). Sixty-three percent (22 cases) of all measurements were taken during mechanical ventilation–all of them under continuous sedation with a mean RASS score of −3 (IQR from −2.8 to −4.1). Five (5) patients (6.25%) received therapy with alpha-adrenergic vasoactive drugs. The mean VIS score was 8 (RIQ 0–10).
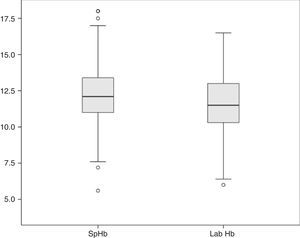
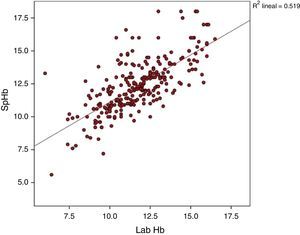
The average Hb concentration exhibited in the core lab analyser was 11.7±2.05g/dl (median 11.5, IQR 10.27–13). The average Hb concentration exhibited in the pulse-oximeter was 12.32±2g/dl (median 12.1g/dl, IQR 11–13.4) (Fig. 1). The Pearson correlation coefficient was 0.72 (p<0.001), and the coefficient of determination R2, 0.52 (Fig. 2). The overall ICC was 0.69 (95% CI: 0.55–0.78), p<0.001. The ICC in determinations with PI>1.5 was 0.70 (95% CI: 0.6–0.78), but when the PI was ≤1.5, the ICC was 0.68 (95% CI: 0.43–0.81)–being both values statistically significant (p<0.001). Concordance was higher in individuals over 6months (0.7, 95% CI: 0.59–0.78) compared to individuals under 6 months (0.59, 95% CI: 0.36–0.74). When the monitor exhibited low signal quality (12.7% of cases), the ICC was 0.45 (95% CI: 0.06–0.71), while in the remaining cases the ICC was 0.74 (95% CI: 0.62–0.81).
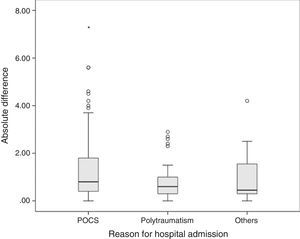
The average difference between lab values and pulse co-oximeter values was 0.66±1.46g/dl, with a median of 0.5g/dl (IQR from −0.2 to 1.4). The mean difference in absolute values was 0.8g/dl (IQR from 0.4 to 1.7). The mean difference between both methods was greater in postoperative cardiac surgery patients (0.8g/dl, IQR from 0.4 to 1.8) than in polytraumatized patients (0.6g/dl, IQR from 0.3 to 1) and in the remaining patients (0.45g/dl, IQR from 0.3 to 1.57), though these differences were not statistically significant in the Kruskal–Wallis test (p=0.065) (Fig. 3).
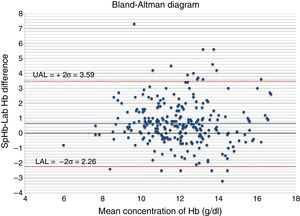
The Bland–Altman diagram (Fig. 4) shows that most differences can be found within the concordance limits – being the upper limit (+2DE) 3.59g/dl, and the lower limit (−2DE) −2.26g/dl. As it can be seen in Table 1, 60.9% of all measurements taken with the non-invasive pulse co-oximeter exhibited a ≤−1g/dl difference with respect to data exhibited by the core lab analyser.
Using a linear regression model, the correlation between the absolute difference of both measurements and the real Hb concentration could be studied, and a statistically significant correlation was found (p=0.007), with a β coefficient of −0.09 and a coefficient of determination R2 of 0.027.
In the multivariate linear regression analysis, the absolute difference between both methods was used as the dependent variable, including the variables that had a statistically significant relation in the correlation matrix as well as race, reason for hospital admission and need for invasive mechanical ventilation. Using a backward step-wise proceeding, the multivariate model was built, maintaining both all significant variables and those variables enhancing the validity of the model as Table 2 shows. According to this model, the lower the Hb concentration is, the higher the HR and fetal Hb concentration are; the difference between both methods of measurement becomes greater in a statistically significant way.
Multivariate linear regression analysis. Dependent variable: absolute difference between SpHb and Hb in the core lab.
| Model | Unstandarized coefficients | t | p | 95% CI for B | ||
|---|---|---|---|---|---|---|
| B | Typical error | Lower limit | Upper limit | |||
| (Constant) | 4.884 | 1.706 | 2.863 | 0.005 | 1.525 | 8.244 |
| Lab Hb (g/dl) | −0.180 | 0.036 | −4.979 | 0.000 | −0.251 | −0.109 |
| Age (months) | −0.003 | 0.002 | −1.741 | 0.083 | −0.006 | 0.000 |
| HR (lpm) | 0.009 | 0.003 | 2.747 | 0.006 | 0.003 | 0.016 |
| RT (°C) | −0.074 | 0.045 | −1.636 | 0.103 | −0.164 | 0.015 |
| Fetal Hb (g/dl) | 0.064 | 0.032 | 1.989 | 0.048 | 0.001 | 0.127 |
HR: heart rate; Hb: hemoglobin; RT: rectal temperature.
One multivariate logistic regression analysis (Table 3) was carried out in order to determine the variables that could be associated with a greater risk of exhibiting low signal in the monitor and, therefore, lower correlation between the real value and the one exhibited by the Radical7 station, and it was found that the lower the PI and rectal temperature are, the higher the risk of low signal quality is (OR 0.531 [95% IC: 0.32–0.88] and 0.529 [95% IC: 0.33–0.85], respectively).
Multivariate logistic regression analysis. Dependent variable: low signal quality.
| p | β | OR | 95% CI for the OR | ||
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Age (months) | 0.388 | 0.005 | 1.005 | 0.944 | 1.016 |
| SpO2 | 0.105 | −0.058 | 0.943 | 0.879 | 1.012 |
| HR (lpm) | 0.101 | 0.019 | 1.019 | 0.996 | 1.043 |
| Lab Hb (g/dl) | 0.412 | −0.091 | 0.913 | 0.734 | 1.135 |
| PI | 0.015 | −0.632 | 0.531 | 0.320 | 0.884 |
| RT (°C) | 0.009 | −0.637 | 0.529 | 0.327 | 0.855 |
HR: heart rate; Hb: hemoglobin; PI: perfusion index; OR: odds ratio; RT: rectal temperature.
Chi square (or Hosmer–Lemeshow) test=7.37 for eight degrees of freedom (p=0.497).
AIC=160.15.
This paper shows that the degree of concordance between the SpHb and the hemoglobin concentration obtained using the core lab analyser in patients admitted to the hospital PICU and at risk of bleeding is good and meets the manufacturer's technical specifications. However, we found wide limits of concordance. Very few pediatric studies have analyzed the precision and concordance of this innovative method of transcutaneous measurement and most have been conducted in hemodynamically stable patients, or patients at low risk of bleeding.12–14 Most papers published so far discuss adult patients,5,16,17 in whom the technology based on multiple wavelength absorption spectroscopy achieves better results probably due to the characteristics of the explored area, the lower heart rate and the higher systolic volume with respect to children.
Phillips et al.18 recently published the results of one prospective study–similar to this paper, and conducted among children hospitalized at one PICU; they found that the average difference between both methods was lower than the one reported in our series (0.07g/dl); however, the limits of concordance are wider than the ones found in our paper (from −5.11 to +5.25g/dl) and the Pearson correlation coefficient was lower (r=0.55). This difference may be partially due to the smaller size of the sample (53 patients, 83 measurements), since, on the other hand, they show a difference percentage ≤1g/dl (67% versus 61% in our series) similar to ours.
Among the factors that may affect the precision of transcutaneous monitoring we find that the total Hb concentration, the HR and the fetal Hb concentration increase the difference with respect to the determination obtained in the core lab. On the other hand, the lower the temperature and PI values are, the more likely it is to achieve low signal qualities in the Radical7 station. In the manufacturer's technical documentation, hemoglobinopathies are described as a potential cause of errors when reading the hemoglobin concentration,11 which might explain the influence of the fetal Hb concentration on the difference seen in the core lab concentration. Although anemia is not specifically mentioned, other authors also found worse correlations with extreme values of Hb.18,19 Most pediatric studies published so far have been conducted in healthy or hemodynamically stable patients – being this is the reason why the effect of low values of Hb, high values of HR or peripheral hypoperfusion has not been extensively studied yet.12,13,20,21 The proportion of cases where no measurements could be taken even after three (3) attempts (5.2%) is similar to the numbers found in medical literature,22,23 where percentages range from 2.6 to 12.3%. The fact of not being able to obtain SpHb measurements may be due to technical errors since yet despite the training of all the staff from the PICU in the correct placement of sensors and use of the device, the same clinician was not present in all the measurements. Nevertheless, in our opinion, the results from this paper show the utility of this device in daily clinical practice.
The degree of concordance between the SpHb and Hb concentration exhibited by the core lab analyser shows the clinical utility of this device even though its exact role is not well defined yet. Two (2) recent meta-analysis mainly based on studies conducted in adult patients state that this technology is not accurate enough to be used as the sole indicator of transfusion.24,25 However, in studies that tested this method in surgical patients, this device seemed to be useful for monitoring the tendency of Hb values, optimize the moment to perform blood sample analysis, and avoid unnecessary venipunctures.12,13,15 On this regard, it would be interesting to conduct cost-effectiveness studies to assess the utility of this non-invasive method from this perspective.
ConclusionsThe estimate of the Hb concentration using the non-invasive pulse co-oximetry shows good global correlation with the Hb concentration using the core lab analyser, though with wide limits of concordance. In our setting, the main advantage is the possibility of continuous non-invasive monitoring of the tendency in patients at risk of bleeding. The reliability of this method is limited in cases of poor peripheral perfusion.
FinancingThis paper has not received any financial support.
Conflicts of interestWe the authors declare that while conducting this paper there were no conflicts of interests linked whatsoever.
The authors of this paper wish to thank the help and collaboration from the nursing and medical staff at the Pediatric Intensive Care Unit without whose kind support we could have not conducted this study.
Please cite this article as: García-Soler P, Camacho Alonso JM, González-Gómez JM, Milano-Manso G. Monitorización no invasiva transcutánea de la concentración de hemoglobina en pacientes críticos pediátricos con riesgo de sangrado. Med Intensiva. 2017;41:209–215.