Neurosurgical patients frequently require admission to intensive care units, either for postoperative management or for treating complications. Most neurosurgical diseases require specific monitoring and prophylaxis. The basic principle of neurosurgical patient management is to ensure correct brain tissue perfusion, i.e., maintaining a sufficient blood flow to supply energy and oxygen to the brain parenchyma. In the last few years, several systems have been developed and improved for monitoring variables such as intracranial pressure, cerebral electrical activity (electroencephalography), cerebral blood flow, parenchymal oxygenation (tissue oxygen pressure) or locoregional metabolism (microdialysis). The present study provides an overview of the general management of neurosurgical patients and the main complications that may occur during the postoperative period. An interventional algorithm is also proposed to facilitate physician decisions, with the inclusion of multimodal neuromonitoring.
Los pacientes con patología neuroquirúrgica requieren frecuentemente el ingreso en unidades de cuidados intensivos tanto para su manejo en el postoperatorio inmediato como para el control de las complicaciones que puedan presentar. La patología neuroquirúrgica es amplia y requiere profilaxis, tratamiento y monitorización específica. El tratamiento del paciente neuroquirúrgico se basa en asegurar una correcta perfusión tisular cerebral, es decir, mantener un flujo sanguíneo suficiente para aportar energía al parénquima cerebral. Con el objetivo de optimizar el tratamiento y el manejo de estos pacientes, en los últimos años se han desarrollado y perfeccionado diferentes sistemas para monitorizar variables como la presión intracraneal, la actividad eléctrica cerebral (electroencefalografía), el flujo cerebral, la oxigenación del parénquima (presión tisular de oxígeno) o el metabolismo locorregional (microdiálisis). Esta revisión sintetiza el manejo general del paciente neuroquirúrgico así como el de las principales complicaciones que puede desarrollar durante el postoperatorio. Asimismo, se propone un algoritmo de actuación para facilitar la decisión de los profesionales responsables que incluye la neuromonitorización multimodal.
Patients with neurosurgical conditions represent a great number of admissions to intensive care units (ICU) since they include traumatic brain injuries (TBI), spontaneous hematomas, ischemic strokes, subarachnoid hemorrhages (SAH) or elective surgeries.1 In general, the immediate postoperative management of these patients is based on maintaining good cerebral tissue perfusion, that is, keeping a proper cerebral perfusion pressure (CPP), while making sure that the brain is properly oxygenated.2
The indication for ICU admission may include the postoperative admission following an intervention, altered state of consciousness requiring clinical or multimodal monitoring, need for mechanical ventilation (MV) to protect the airways or the appearance of postoperative medical complications such as epileptic seizures, infections, pulmonary embolisms, etc.3 We should mention here that although recent studies question the ICU admission of all neurosurgical patients,4 it has been confirmed that admissions to specialized ICUs have the best final outcomes.5
Neurosurgical complications may be fatal and include infections of the surgical wound/postoperative meningitis, intraparenchymal hemorrhage, and epileptic seizures. To avoid developing complications, we have to pay special attention to the specific peculiarities of the antibiotic prophylaxis of surgery, sedation and analgesia, the strategy of MV, and the specific multimodal monitoring when needed.
Postoperative careClinical and radiologic monitoringWe will perform a thorough clinical examination while paying special attention to the level of consciousness according to the Glasgow coma scale (GCS), the size of the pupils or the FOUR score.6
If the patient shows sudden and/or maintained changes during neurological examination, we should reassess the need to perform imaging modalities such as a computed tomography scan or a magnetic resonance imaging in search for any treatable complications.7,8
Respiratory monitoring and need for mechanical ventilationNeuronal dysfunction is one of the leading causes for needing MV.9 The goal of using MV in patients is maintaining a patent airway in patients with low levels of consciousness due to the risk of aspiration and eventually avoiding hypoxemia and hypercapnia.
Ventilated neurological patients usually require longer ICU stays, have a higher tracheostomy ratio, and use PEEP therapy for shorter periods of time.10
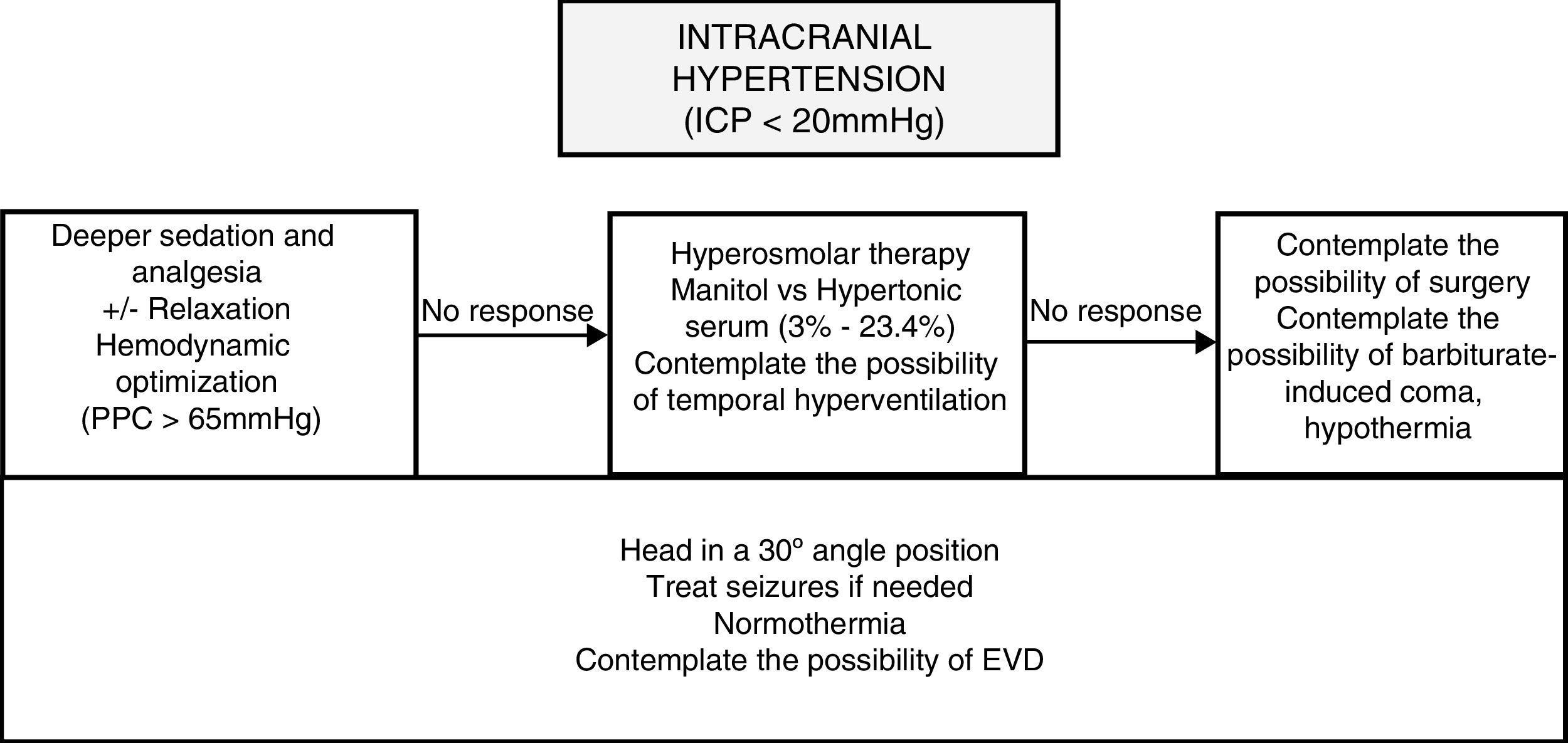
Traditionally, the levels of PEEP (≤5cmH2O) are maintained low to avoid increasing intracranial pressure (ICP), but recent studies suggest that it is safe to use higher levels of PEEP since this higher levels improve the oxygenation of the brain.11 On the other hand, to this day, it is not recommended to keep prophylactic hyperventilation for long periods of time to reduce pCO2,12 but it can be used as a temporary measure to reduce documented and monitored intracranial hypertension (ICH).
Sedation and analgesiaThe need for deep sedation has been proven to increase the number of days on MV, delirium, and mortality in patients admitted to the ICU.13–15 The actual clinical guidelines on the management of sedation at the ICU setting recommend, in the absence of contraindications, the use of a strategy of mild sedation and prioritize the use of analgesia and non-benzodiazepine drugs.16
In neurosurgical patients it is important to conduct optimal neurological examinations and in this sense, it is necessary to use sedation and analgesia to be able open neurologic windows frequently being mild sedation and analgesia safe in this subgroup of patients.17,18
Fluid therapy and electrolytic alterationsFluid therapy is usually divided into crystalloids and colloids. Crystalloids have small soluble molecules and can be divided into two subgroups: saline solutions and balanced solutions. The latter help to achieve isotonic osmolarity; they have different ions depending on their type, but all of them have fewer chlorine: the excess of exogenous chlorine has been associated with an increase of hyperchloremic metabolic acidosis, renal dysfunction, GI dysfunction, and the secretion of inflammatory cytokines.19 Ringer's Lactate solution has less osmolarity compared to all the other crystalloids, which is why large infusions with this crystalloid can increase brain swelling and ICP.20 For resuscitation purposes and for the very maintenance of the neurosurgical patient, we should use isotonic solutions but always avoid hypotonic solutions.21,22
Colloids have larger molecules with less capacity to spread through semipermeable membranes. Today we use crystalloids more than we use colloids since no significant differences in mortality have been reported with the use of colloids compared to the use of crystalloids.23
Hypertonic solutions are used for the management of ICH to reduce brain swelling by drawing water from the nervous tissue through osmotic pressure to the interstitium. No differences between hypertonic sodium solutions and mannitol have been reported in the management of ICH.24
Hyponatremia is the most common electrolytic disorder in neurosurgical patients. The most common conditions that lead to hyponatremia are the inadequate secretion of the antidiuretic hormone and the so-called cerebral salt wasting brain syndrome (CSWS). Both present with increased hyponatremia and natriuresis being the main difference here extracellular volume (ECV) since the syndrome of inappropriate antidiuretic hormone ADH release (SIADH) presents with a normal ECV, while the CSWS presents with a reduced ECV. Therefore, the main differentiating factor here is circulating blood volume. In the presence of symptoms of severe hyponatremia it is advisable to infuse one hypertonic saline solution at 3%.19,25,26
We can also have sodium disorders in the form of hypernatremia. Insipid diabetes is characterized by dehydration with hypernatremia following a reduction of the antidiuretic hormone. Here management is based on hydration and use of ADH analogs such as desmopressin or vasopressin.20
Postoperative prophylaxisAntibioticThe prevalence of surgical wound infection is between 1% and 11%.27 Due to the risk involved in a neurosurgical infection, antibiotic prophylaxis in neurosurgical procedures is widely accepted.28,29
The election of the antibiotic to use tries to cover the most commonly isolated germs: Staphylococcus aureus and plasmocoagulase-negative staphylococcus. For this kind of prophylaxis, the options here are cotrimoxazol or cefazolin. For surgeries of the back of the skull through trans-nasal and/or trans-oral access, antibiotic prophylaxis with amoxicillin-clavulanic acid is recommended.
In patients with TBI, antibiotic prophylaxis is advisable for the management of open skull fractures. Different regimens have been suggested: amoxicillin-clavulanic acid or cefuroxime with metronidazole.30–32
In closed fractures of the base of the skull, the use of antibiotics does not seem to alter the incidence of CNS infections. In the presence of an associated CSF fistula, evidence on the utility of antibiotics is weaker here.33–35
In sensors of invasive neuromonitoring such as the ICP sensor, the tissue oxygen pressure (PtiO2) sensor, and the microdialysis sensor, there is no evidence supporting the use of antibiotics during the insertion time of these devices.36–38
When placing external ventricular drainage, the IV treatment administered during the drainage insertion time is not recommended due to the risk of increasing antibiotic resistance. Still, a dose prior to insertion is advisable.39
In spinal surgeries, antibiotic surgical prophylaxis is indicated; the antibiotic of choice should be picked based on each particular case, comorbidities, and risk factors. Once again cefazolin is the antibiotic of choice here.40
Preventing seizuresThe use of prophylactic antiepileptic drugs in neurosurgical procedures is controversial in neurosurgical conditions and there is not too much evidence on this regard.
Although up to 60% of the patients with a brain tumor can have seizures during the progression of the disease,41 the use of antiepileptic drugs has not proven capable of reducing the onset of epileptic seizures in these patients42 and its routine use is not recommended.21,43
In patients with severe brain traumas, the use of phenytoin is recommended to reduce the incidence of early post-traumatic epileptic seizures. Although the use of levetiracetam has been suggested, there is not enough evidence at the moment to recommend it over phenytoin in this subgroup of patients.12
Despite the high incidence of epileptic seizures in patients with SAH (between 4% and 26% when the hemorrhage occurs),44 the systematic use of antiepileptic drugs is not recommended for the management of SAH. Recent systematic reviews conclude that there are no pieces of evidence to support or refute the use of antiepileptic drugs for primary or secondary prevention purposes of SAH-related seizures.45,46
Given the baseline pathological disparity that ends up leading to performing craniotomy procedures, there is little evidence on whether the use of antiepileptic drugs when performing craniotomy procedures should be recommended.47
The clinical guidelines for the management of strokes recommend the prophylactic use of antiepileptic drugs but only in the presence of seizures. A Cochrane review48 concludes that no beneficial effects were seen after comparing valproic acid with placebo for the primary prevention purposes of seizures that follow a brain hemorrhage.49
Antithrombotic prophylaxisThromboprophylaxis should be prescribed individually on a per-case basis and always bearing in mind the risk factors of thromboembolisms,50,51 such as underlying conditions. Recent studies suggest that the use of pharmacological prophylaxis does not increase significantly the risk of brain bleeding.52
We can make a distinction here between mechanical thromboprophylaxis with intermittent pneumatic compression measures and pharmacological thromboprophylaxis with subcutaneous low molecular weight heparin or subcutaneous unfractionated heparin.50
If we focus on neurosurgical conditions only, the clinical guidelines recommend the early use of mechanical thromboprophylaxis and, if possible, change to pharmacological thromboprophylaxis. Still both approaches are associated with high risk factors such as ischemic strokes and spinal injuries. In the management of non-traumatic intracranial hemorrhages, it is recommended to delay the use of pharmacological thromboprophylaxis for 48h after admission, after normalizing coagulation, and as long as the hematoma remains stable. In the case of TBIs with hemorrhages the recommendation is to use mechanical thromboprophylaxis for up to 24–48h and then evaluate whether it is possible to change to pharmacological thromboprophylaxis. In patients with SAH it is also recommended to start with mechanical thromboprophylaxis at hospital admission, and then change to pharmacological thromboprophylaxis after securing the aneurysm. In patients undergoing elective craniotomy procedures it is recommended to initiate pharmacological thromboprophylaxis within the next first 24h.50,53
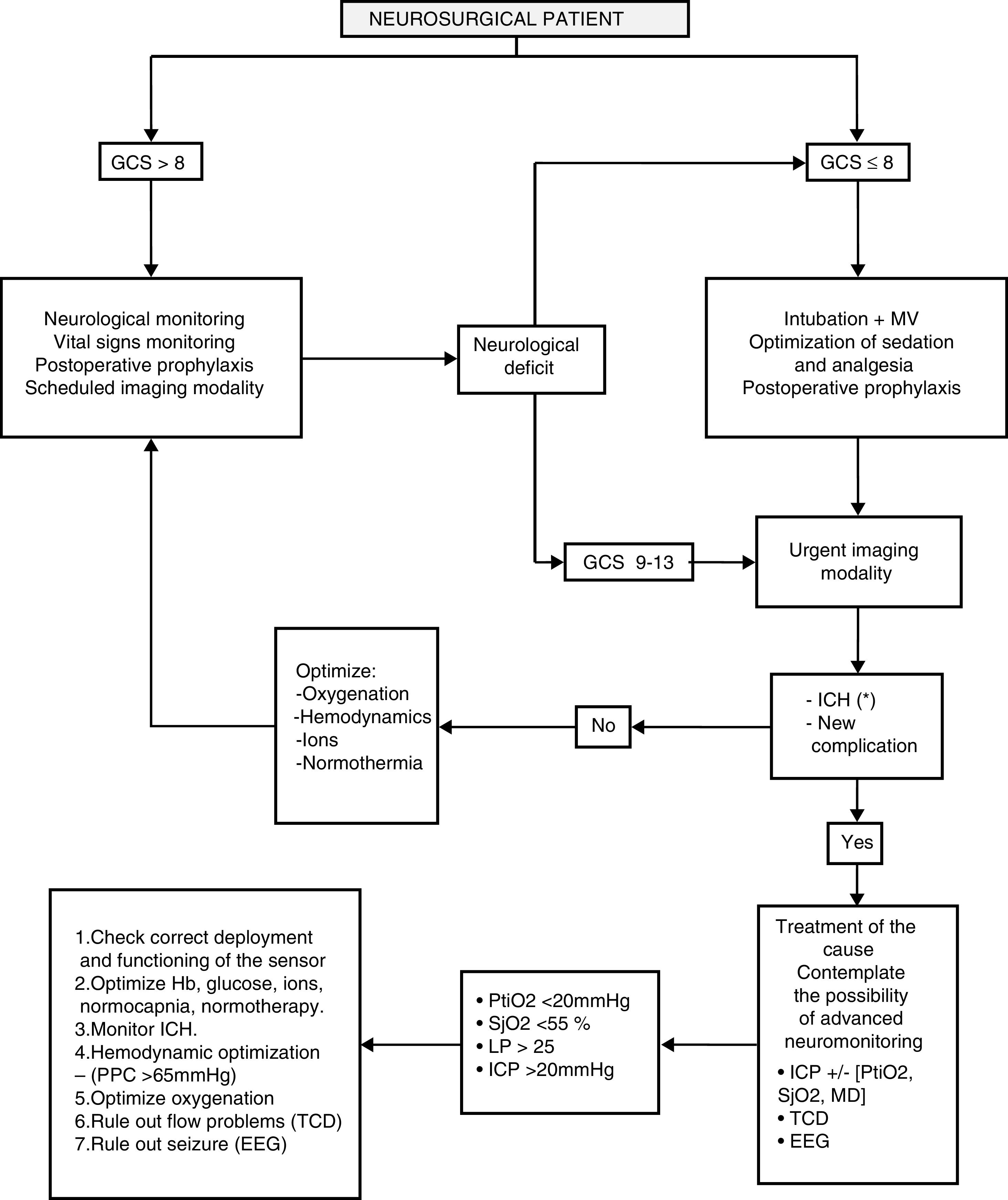
NeuromonitoringWhen neurological examination is not possible or it provides insufficient information, then the possibility of advanced neuromonitoring will be assessed. The neuromonitoring algorithm is shown in Fig. 1.
General management of the neurosurgical patient.
CPP, cerebral perfusion pressure; EEG, electroencephalogram; GCS: Glasgow Coma Score; ICH, intracranial hypertension; ICP, intracranial pressure; LP, lactate-pyruvate; MD, microdialysis; MV, mechanical ventilation; PtiO2, tissue oxygen pressure; SjO2, oxygen saturation in the jugular bulb; TCD, transcranial Doppler.
* See Fig. 2.
Monitoring ICP is essential in the management of patients with diffuse brain injuries and the increases of ICP seen have been associated with more mortality.54–56 Normal ICP levels are between 5 and 15mmHg.57 Usually levels >20–25mmHg are considered an indication for ICP monitoring. The recommendations established to monitor ICH are shown in Fig. 2.
It is advisable to measure ICP in patients with TBI with initial GCS ≤8 and an abnormal CT scan, and also in patients with normal CT scans but over 40 years of age, arterial hypotension (systolic blood pressure <90mmHg) or abnormal motor responses to pain.58
Monitoring ICP is also recommended in comatose patients with contusions or who cannot be clinically examined or in whom withdrawing sedation can be dangerous, and also after performing secondary craniotomy procedures. Also, the ICP should be taken into account in patients with acute supratentorial hematoma with risk of ICH.57,59
Starting with the ICP we can obtain the CPP, defined as mean arterial pressure—ICP.60 The goal here is to try to keep CPP >60mmHg; lower levels are associated with worse final outcomes.61
Monitoring ICP also allows us to objectify brain self-regulation, that is, the capacity of the brain to perform vasoconstriction with increased arterial pressure. The mean velocity index based on the CPP and the index of self-regulation index (ISR) both predict mortality and the final neurological outcome of the TBI and the ISR, the neurological outcome of SAH.62
Cerebral blood flowWe can determine the cerebral blood flow through invasive measures such as thermal diffusion flowmetry. The main setback of this technique is that it is a regional measuring system that provides limited information of a parenchymal region sampled.63
The use of the color Doppler and the transcranial Doppler ultrasonography to estimate the blood flow of larger areas of the brain has proven useful especially in the management of SAH for the semi-continuous monitoring of late ischemic deficit and vasoespasm.45,64 Still, not all patients have a good acoustic window and accuracy here largely depends on the skills of the operator.57
Cerebral oxygenationAnother pillar of cerebral perfusion is the correct oxygenation of the brain and to know what systemic oxygenation looks like we can use the pulse oximetry and then measure the etCO2 to avoid hyperventilation and secondary cerebral vasoconstriction.57
The PtiO2 gives the continuous local saturation of the parenchyma being the normal values somehow between 20 and 35mmHg.65 There is a correlation between low PtiO2 levels and morbimortality, and levels below 20mmHg are considered an indication for therapeutic management.66 Levels <20mmHg are indicative of compromised oxygenation and treatment should be considered here to optimize the oxygen supply.57
The SjvO2 gives us global information on the use of brain oxygenation. It is measured using one catheter that is placed inside the dominant internal jugular vein on the edge of the jugular bulb.67 Values between 55% and 75% are considered normal. Values <55% are indicative of risk of ischemia and values >75% are suggestive of hyperemia, lower metabolism or cellular death.68–70
Using the PtiO2 sensor and the SjvO2 sensor is recommended in patients with risk of ischemia.12,57,71
Using near infrared spectroscopy (NIRS) we can estimate the oxygen saturation of brain tissue.72 Its use is not recommended by guidelines on neuromonitoring due to the little evidence available and the limitations of this technique in adult patients.57,73
Cerebral metabolismThe poor tissue perfusion of the cerebral parenchyma alters extracellular homeostasis which can be measured using one microdialysis catheter.74 Usually the levels of lactate, pyruvate, glucose, glutamate, and glycerol are measured here in order to determine the presence of metabolic crisis.74
Glucose is the main source of energy for the brain. Maintained drops of glucose values at brain level (<0.8mM) have been associated with worse prognostic results following severe TBI and SAH.74
In normal conditions, glucose is metabolized into pyruvate generating ATP in a pathway called the Krebs cycle; however, in situations of hypoxia or mitochondrial dysfunction, pyruvate is metabolized into lactate. An increase of the lactate/pyruvate (LP) ratio in the extracellular space translates into a situation of hypoxia or neuronal dysfunction. An increase of the LP ratio with drops of the pyruvate levels is indicative of classic ischemia; however, an increase of the LP ratio with normal levels of pyruvate is suggestive of non-ischemic causes such as mitochondrial dysfunction.75
Glutamate has been associated with cellular damage and inflammatory response. High glutamate levels are indicative of hypoxia and/or ischemia. Glycerol is one lipidic component of neurons and a marker of neuronal cellular damage which is why high glycerol levels are a strong marker of hypoxia and/or ischemia and destruction of the neuronal membrane.75
The use of microdialysis is recommended in patients with risk of brain ischemia, hypoxia, and situations of glucose deficit.64,76
Cerebral electrical activityThe electroencephalogram (EEG) registers the electrical activity and it is essential for the detection of epileptic seizures, especially the non-convulsive ones. The continuous EEG (cEEG) is more sensitive for the detection of non-convulsive epileptic seizures compared to conventional EEG,77 but the existing technical and interpretation difficulties and other availability issues have limited the widespread use of this technique.
The cEEG is indicated in patients with persistent or unexplained altered states of conciousness.57 The EEG is useful for the diagnosis of the non-convulsive status epilepticus but also to assess the response to therapy. Similarly, in patients with high-grade SAH, the cEEG can reveal late-onset ischemic neuronal deficits.
Conclusions- –
Neurosurgical patients require comprehensive postoperative management including dynamic monitoring based on their clinical situation.
- –
In patients who remain conscious, complete routine neurological examinations should be performed to detect the appearance of deficits misdiagnosed during the ICU admission.
- –
Neurological patients require ventilation strategies and analgesia and sedation to promote the oxygenation of the brain and to limit periodic windows of sedation.
- –
In sedated patients in whom a complete clinical examination is not possible, multimodal monitoring should be taken into serious consideration including measuring devices such as ICP, PtiO2, EEG to optimize management and detect the appearance of complications.
The authors declared no conflicts of interest whatsoever related to this manuscript.
Please cite this article as: Santafé Colomina M, Arikan Abelló F, Sánchez Corral A, Ferrer Roca R. Optimización del manejo del paciente neuroquirúrgico en Medicina Intensiva. Med Intensiva. 2019;43:489–496.