Patient care after major head and neck surgery has changed in recent years. Tumors are the most common reasons for this type of surgery, though it is also used to treat benign conditions.
Recent advances in equipment and surgical techniques have improved the postoperative course in this field, allowing early recovery, less pain and infection, a shorter hospital stay, and even better aesthetic results. This is due to the use of minimally invasive techniques, which are gaining relevance. Such techniques allow complex procedures in the head and neck region, through natural orifices or small incisions, with minimal damage and sequelae for the patients.
Despite these advances, however, the complexity of the treatment intervention requires multidisciplinary patient management, mostly in the Intensive Care Unit, in order to monitor the possible occurrence of complications. Potential risk factors include previous comorbidity, the type of surgery involved (e.g., bilateral cervical lymphadenectomy), multiple transfusions, and the appearance of early complications requiring repeat surgery.
Despite the existence of several studies, there are no standardized protocols for the postoperative period in surgeries of this kind. This causes many specialists to resort to accelerated recovery protocols (ERAS: “Enhanced Recovery After Surgery”) that have already been established in other surgical specialties.
El cuidado del paciente tras cirugía mayor de cabeza y cuello ha cambiado en los últimos años. La patología que acapara este tipo de intervenciones es la tumoral; aunque también se utiliza para resolución de patologías benignas.
Recientes avances en el equipamiento y en las técnicas quirúrgicas han mejorado el postoperatorio en este campo, permitiendo una recuperación precoz, menor grado de dolor y tasa de infección, menor estancia hospitalaria e incluso mejores resultados estéticos. Esto se debe a las técnicas mínimamente invasivas, que están cobrando protagonismo en los últimos años. Éstas permiten realizar procedimientos complejos en la región de cabeza y cuello, a través de orificios naturales o pequeñas incisiones, con mínimo daño y mínimas secuelas para los pacientes.
A pesar de estos avances, el manejo de estos pacientes dada la complejidad de la intervención, requerirán un manejo multidisciplinar, la mayor parte de ellas en las Unidades de Cuidados Intensivos (UCI) para vigilar la posible aparición de complicaciones. Entre los factores de riesgo potenciales destacan, comorbilidad previa, el tipo de intervención, como el vaciamiento cervical bilateral; necesidad de politransfusión y aparición de complicaciones precoces que requieren nueva reintervención.
A pesar de diversos estudios no existen protocolos estandarizados para el período postoperatorio de este tipo de intervenciones, lo que hace que muchos trasladen protocolos de recuperación precoz (ERAS: “Enhanced Recovery After Surgery”) ya instaurados en otras especialidades quirúrgicas.
Although there is a percentage of patients who undergo head and neck surgery (HNS) who may not require admission in special units, a few hospitals have High Dependency Units or goal-directed resuscitation units available lead by anesthesiologists; even some patients are directly hospitalized. Admission in the intensive care unit (ICU) in patients undergoing complex techniques or with comorbidities that give rise to higher chances of sustaining postoperative complications that may require close monitoring.1 As Vosler et al.2 say in their study, a useful tool would predict surgical risk and factors predisposing to complications after oncological head and neck surgery.
Kovatch et al.3 describe that 75.2% of patients admitted to the ICU in the immediate postoperative of microvascular surgery with tissue graft spend, on average, 2.5 days at this unit. In this case, and yet despite the usual monitoring of complications during the standard postoperative period, it is essential to keep close monitoring and examination of the state of the graft. That is why during the first 48h a high percentage of these patients require ICU admission. Other studies show higher percentages of patient admission; it all depends on multiple factors such as the center policy, the more or less frequent monitoring needs, the amount of this type of annual interventions, and cost rises associated with admissions in these units.4
We may agree on a series of general indications for bed requests or ICU admissions in the immediate ENT and NHS postoperative periods like the need for advanced respiratory support or support due to organ failure in patients with underlying comorbidities (Table 1). Patients with organ failure other than respiratory failure or whose general situation does not make them eligible to be moved to the hospital floor unit would benefit from closer monitorization in intermediate care units (Table 2).5 In our country, as it is the case with our center, the lack of other specific units leads to managing these potoperative periods primarily at the ICU.
In particular, facial fractures in polytrauma patients—the major oncological reconstructive surgery of head and neck—skull base surgeries, and all clinical situations that may compromise the airway and cause cervico-facial postoperative bleeding are clear examples of multidisciplinary care at the ICU. Also, good communication among different specialties is essential for a good overall management of the patient.
Tables 3 and 4 show ENT and HNS surgical processes eligible for ICU admission. The ICU stay will depend on the patient progression. In scheduled surgeries like previously reported by Kovatch et al.3 ICU stays go from 24h to 72h; in patients requiring urgent reinterventions in the immediate postoperative period or needing it without having received a prior intervention, the ICU stay will vary depending on the underlying condition, type of intervention, organ damage in this context and support from the required ICU, intercurrent infections, and prior comorbidities.
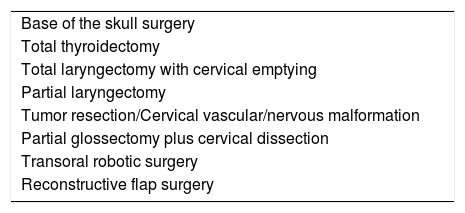
Surgical ENT conditions with criteria for ICU admission.
| Base of the skull surgery |
| Total thyroidectomy |
| Total laryngectomy with cervical emptying |
| Partial laryngectomy |
| Tumor resection/Cervical vascular/nervous malformation |
| Partial glossectomy plus cervical dissection |
| Transoral robotic surgery |
| Reconstructive flap surgery |
Maxillofacial surgery conditions with criteria for ICU admission.
| Infectious (odontogenous, Periamygdaloid abscess, Ludwing angina) |
| Facial traumas |
| Dentofacial deformity/Bimaxillary orthognatic surgery |
| OSAHS/uvulopalatopharyngoplasty, orthognatic |
| Oncological head and neck surgery, reconstructive |
| Glosectomy/Hemiglosectomy |
| Complex craniofacial reconstructions, tumors at the base of the skull with immediate reconstruction |
| Craniofacial malformations, central facial fissures, rhinoplasties, palatoplasties, orthognatic surgeries, facial rehabilitations, and reconstructions |
OSAHS, obstructive sleep apnea-hipopnea syndrome.
Over the last decade, robotic techniques have revolutionized minimally invasive surgery exceeding the advantages of any standard surgical procedure. It consists of improving the computer technology of a device to apply it to a surgical procedure involved in doctor-patient interaction. Such a device assumes some degree of control only reserved to surgeons so far. In the field of ENT and NHS surgeries these techniques have become widely used in the management of malignant and benign conditions alike. Transoral robotic surgery (TORS) has become a very important pillar of this surgical specialty.6
These are some of its advantages: 3D visualization with image magnifying capabilities to obtain higher definition, depth, and dimension of structures; elimination of the surgeon’s tremors and physical fatigue allowing more precise moves in a comfortable position for the surgeon during the procedure. It includes several multi-jointed robotic arms and these qualities can be used remotely through videosurgery. But there are some limitations too since both the material and the instrumentation are expensive. Also, installation and maintenance, and the enormous size and weight of the equipment require readjusting the espace usually occupied by the medical staff at the OR, which means that proper space is required. Its use requires learning curve and it eliminates the human component; in this case no surgeon touches the tissue he is operating on.7
Aspects to remember in the postoperative of ENT and HNS surgeriesThe complexity of these procedures requires fully-coordinated multidisciplinary management for preoperative preparations, intraoperatorive management, and for the postoperative care of these patients. Many centers provide an excellent management of this type of patients but there is still no consensus on what the action guidelines on this regard should be, and variations in perioperative care is still significant. Several studies8 have compared the effectiveness of Enhanced Recovery After Surgery (ERAS) protocols whose benefits were less mortality and days of hospital stay. Initially they were developed for colorectal surgery; a series of recommendations have been extrapolated to other surgical specialties. According to the review conducted by Dort et al.9 the implementation of early recovery protocols (ERAS guidelines) reduces the incidence of surgical complications, hospital stays, and costs of different surgical disciplines. It consists of several recommendations starting in the preoperative period with presurgical training based on preparing the patients and the family members. Also, it establishes the eating habits prior to the procedure, antithrombotic and antibiotic prophylaxis, and intraoperative management through anesthesia until the close monitoring of the graft plus tracheostomy care in the postoperative.
AirwayAirway patency is the most important clinical goal in the immediate ICU postoperative of these procedures. In ENT surgeries, the airway can be compromised due to hemorrhage and the formation of asphyctic hematoma. This is usually the case with radical parathyroidectomy, total laryngectomy with cervical dissection, tumor resection surgery, and reconstructive flap surgery. Performing a tracheostomy is essential in some of these surgeries like total laryngectomy with cervical emptying.10
According to Godden et al.11 since orotracheal intubation would be really difficult in these patients (anatomy distortion with impossibility to perform a laryngoscopy or use stylets or laryngeal masks), the obstruction of the tracheostomy could seal the airway leading to a situation of extreme urgency. This possibility would be indicative of ICU management during the first 24h–48h.
Other processes like minimally invasive surgery, glossectomy, supraglottic laryngectomy, and oropharyngectomy require less aggressive surgeries. In most cases a tracheostomy won’t be neccesary. That is why it is important to monitor the aiway and the possibility of bleeding complications that may occur hours after the procedure. When the upper airway collapses it is often associated with soft-tissue edema, postoperative cervical hematoma or intraoral bleeding. We should not forget that irritants can damage the airway and cause laryngotracheal spasm too.
In sum, these are the steps that should be followed to maintain the airway:
- -
If long intubations are anticipated, a tracheostomy should be performed at the OR with monitoring and care at the ICU. The accidental decannulation of a patient is no problem since, unlike the percutaneous technique, surgical tracheostomy allows an early recannulation without losing trajectory. In cases of bleeding through the airway, the airway patency should be secured first through contiuous suction, while assessing the transfusion and amount of hemoderivatives if needed, and prenoticing the surgical unit for any possible urgent procedures or only through close monitoring with conservative management.12
- -
Other patients are extubated at the OR and they need to be closely and continuously monitored at the ICU with oxygen therapy depending on each particular case. Same as it happens with patients undergoing metallic intermaxillary blocking, this technique may be more difficult to perform when extubating than managing carriers of elastic blocking.13 At this point it is important to know what surgical technique was used because depending on the information received by the surgeon we will have to design an action plan on the possible management of difficult airways, which is what usually happens.14,15
- -
If respiratory support is anticipated for a short span of time, the submental route can be an alternative to tracheostomy (in case nasal or intraoral fractures are treated in the same procedure) but always at the operator’s complete discretion.16
- -
A key issue here is to figure out the ideal time to extubate the patient. References on what this ideal time may be are scarce though.17 It can be said that early extubations in the maxillofacial postoperative can be risky since there is no specific marker to quantify the degree of postoperative inflammation or predict any possible complications of the upper airway. Therefore, the patient should be kept on analgosedation and mechanical ventilation within the first 24h following the immediate postoperative to reduce the incidence of respiratory complications. On the other hand, there is another proposal suggested by the fast track school—the early extubation technique—that uses multimodal approach and planning of the postoperative period to not extend tracheal intubation or mechanical ventilation.18 It is only adviseable for teams specialized in this technique highly competent in the management of complications.
In all cases, close monitoring within the first few hours is essential because of the possible complications reported above. The ICU management of these situations is the way to go since these units have qualified fully trained personnel handling situations like these. Patients carrying tracheostomies after a prophylactic or necessary procedure are preferably those who undergo oncological surgeries leading to the immediate reconstruction of intraoral or oropharyngeal structures. However, in certain traumas, cervical surgeries, resections of vascular lesions, infectious disease processes or patients with prolonged intubation periods it can also be necessary. As a general rule, mechanical ventilatory support should be withdrawn as soon as possible and decannulation should be performed in most cases at the hospital floor unit. The tracheostomy care while at the hospital floor is the responsibility of the nursing staff. The complications these patients may experience have already been reported such as obstructions, decannulations, infections, etc., and the nursing staff needs to be trained in this specific care and know the different types of cannulas available and how to handle them. On the other hand, these patients require more dedication, which may imply work overloads at the hospital floor unit. Also, it is essential that these patients receive training and information on self-care.19
Management of postoperative hemorrhage and thrombosisPostoperative hemorrhages should be treated fast and appropriately since the moment they appear. The great cervicofacial vascularization and the presence of major cervical vessels can make surgery harmful or homeostasis insufficient. We should mention here that previously operated or radiated necks have a higher risk of hemorrhage due to existing fibrosis, lack of dissection planes or vascular wall abnormalities.
We should also take into consideration that infraclavicular bleeding or bleeding at the base of the skull can be difficult to control. It is essential to know the analytical parameters of the hemogram and coagulation and the patient’s hemodynamic status. Anticoagulation and antiaggregration can contribute significantly to this complication so we need to make sure that it has been removed on time before the procedure.
Treatment consists of the administration of hemoderivatives. The actual tendency is both restrictive and conservative when it comes to transfusion. According to Abt et al.20 although the transfusion of hemoderivatives is not associated with more morbidity in this kind of patients, there are no significant differences regarding complications or further adverse reactions in patients with lower transfusion thresholds; they report no differences in the length of hospital stay, survival of the graft, lack of medical or graft-related complications when transfusion occurs with hematocrit levels < 21% or 27%. At the same time, coagulation should be taken into consideration and the infusion of prothrombotics be assessed based on the degree and pace of the hemorrhage, and presence of prior drugs. The opinion of the surgeon is key to plan the decision-making process.
On the other hand, the incidence of deep venous thrombosis and pulmonary thromboembolism is very low in ENT and NHS surgical procedures. In their 2011 review, Williams et al.21 estimated that the risk of thromboembolic disease after maxillofacial surgery is somewhere between 0.15% and 1.6%; for this reason, around 70% of healthcare providers admited that they used antithrombotic prophylaxis. In the latest study conducted by Al-Qurayshi et al.22 the prevalence of thromboembolic disease in this type of surgery is around 0.37%. They evaluated associated risks such as the type of surgery, demographic data, prior comorbidity, prior history of thromboembolic disease, etc. Also, the results associated with longer hospital stays and costs from managing patients with thromboembolic disease as the complication in the postoperative of this surgical specialty. They conclude that having this complication during the postoperative increases mortality rate in 4.87% being more common in males with a prior history of thyroid, parathyroid, mandible and maxillofacial bone, mouth, and paranasal sinus surgeries. It has been well-established that thromboprophylaxis plays an important role reducing costs and the frequency of deep venous thrombosis and thromboembolism, yet prophylaxis at hospital discharge is still to be determined. Antithrombotic prophylaxis is used routinely in other surgical specialties like urology, trauma, etc. In ENT and HNS surgeries it is not that common given the lower risk and potential side effects, basically hemorrhagic.
The administration of heparin should be individualized and indicated in cases of immobilization and also agreed with the surgical team. In our center it is performed after 12h to not promote or interfere with the hemorrhagic complications that may occur at this point.
Circulatory supportOur goal here is the neutral balance of fluids, mainly crystalloids, while keeping the status of normovolemia and normotension to achieve a diuretic rhythm of 0.5–1mL/kg/h. Hypervolemia is associated with poorer results and should be avoided to prevent graft edema. If proven insufficient, vasoactive support with vasopressors may be necessary to secure an adequate perfusion pressure, above all, in reconstructive flap surgeries. The success of this free tissue transference totally depends on continuous arterial and venous flow until the appearance of neovascularization.23 We have hemodynamic monitorization systems that are more or less invasive to obtain the right cardiac output. These systems are more highly recommended with compromised prior cardiovascular functions. The transfusion of hemoderivatives and correction of coagulation abnormalities in case of a hemorrhage is obviously part ot the general hemodynamic optimization of the graft. If the situation makes the patient unstable, it is essential to use imaging modalities and/or an urgent reintervention based on agreed assessment with the specialist.
Analgesia and sedationYet despite the advances made over the last decade on robotic surgery in this field, more and more individualized for each patient, pain is still a basic entity to treat at the OR and, obviously, during the postoperative period. Pain management is crucial to avoid complications and improve recovery.
In patients undergoing ENT and NHS surgeries we can use conventional analgesia in bolus or perfusion with hypnotics and sedatives depending on the level of respiratory control required. The administration of corticoids to treat the edema and pain is a common practice in these procedures, but still needs to be individualized and agreed with the specialist depending on each particular case. There is no action guideline on the use of corticoids and time of administration. In their study Clayburgh et al.24 conclude that long courses of corticoids, 3–4 días, in the postoperative of transoral robotic surgery is safe, facilitates fast recoveries, and reduces hospital stays.
Patients admitted to the ICU are in pain both because of their underlying conditions and the invasive procedures or standard daily procedures they sustain such as mobilizations, aspirations of secretions in tracheostomized patients, healing of wounds, etc. The level of analgesia used should be monitored with the appropriate scoring systems depending on the circumstances of each particular case.25 The analogue visual scale (AVS) for patients who can communicate themselves is one of these scales. For those who cannot give their subjective assessment —considered the gold standard in the assessment of pain—we have several scales but no one universal tool for these situations. These scales are based on observation and the assessment of behavioral indictators such as facial expression, muscle tone, movements or adaptation to mechanical ventilation.26 The ESCID pain scale is used with patients who cannot communicate, has been designed in Spain and includes 5 items: facial expression, tranquility (movements), muscle tone, adaptation to mechanical ventilation and comfortability. The score goes from 0 to 10 and each item recieves a maximum score of 2. When the patient is under sedation the Richmond Agitation Sedation Scale (RASS) is used.
Neurological careWith base of the skull surgeries, we should pay special attention to the possibility of a CSF fistula and cranial neuropathies. The fistula is caused by the condition or due to iatrogenic damage sustained during the procedure. Treatment consists of lower back drainage and targeted antibiotic prophylaxis. It is essential to monitor focality or central or peripheral neurological deficits based on prior lesions and/or techniques used. In cases of resection of neurinoma the region innervated by the facial nerve should be monitored to discard motor lesions or acute vestibular syndrome damage due to damage to CN VIII that can be induced by the procedure. In cases of intracranial hypertension, the appropriate measures should be taken and seizures (reconstructive surgeries and traumas) should be prevented with levetiracetam.27
Skin graft careIn the case of reconstructive flap surgery, the main goal is to secure microvascular anastomosis. If the perfusion of the flap is compromised an immediate procedure can be indicated to maximize the chances of flap viability. Around 50% of all flaps are compromised within the first 4 postoperative hours, and 95% within the first 72h. Therefore, these timeframes should be taken into consideration to establish the time recommended for ICU care. These are the basic recommendations for the use of reconstructive flap surgery technique3,12,23:
- -
Keep the head in a neutral position in the frontal plane avoiding lateralizations or flexions compressing the graft.
- -
Strict hemodynamic control to guarantee vascular perfusion (always >65mm Hg) using fluid therapy and vasopressors to maintain the status of normovolemia and normotension already described in the circulatory support section above.
- -
Serial assessment of graft patency especially within the first 24h–48h. The clinical monitorization techniques consist of evaluating the patient’s color, temperature, capillary filling, adequate turgidity, and bleeding data of the flap. All of it complemented with a color Doppler ultrasound assessment. There are more invasive monitorization modalities available such as microdialysis and fluorometry that are very precise techniques, have high sensitivity, but also expensive and dependent upon an expert to interpret the results.23
The headboard should be kept in a high position to promote the cervical venous drainage compromised by the surgical emptying. Always in the neutral position with the proper care using a nasogastric tube. In cases of craniofacial trauma, a cranial CT scan should be performed to discard fractures at the base of the skull that may lead to the insertion of a nasogastric tube inside the cranial cavity. Even after coming back from the OR it is important to verify the correct intragastric position of this device.
During the ICU stay, patients often remain on restrictive diet. The actual tendency is early nutritional support (within the first 5 days after the procedure).28 Several studies have shown it is a safe practice without a higher percentage of complications compared to late starts (>7 days) that also reduces hospital stay. If there is some level of tolerance, it will normally happen through the nasogastric tube and always on consensus with the surgical expert.
Nausea and vomiting prophylaxis is key especially after reconstructive flap surgery because it can cause graft dehiscence, risk of wound infection, fistulas, etc. The prevention of nausea and vomiting should be considered in all ENT and HNS postoperatives.29
Regarding antibiotic treatment, in clean scheduled or clean-contaminated surgeries, patients will receive the corresponding prophylaxis established by the protocol of each center. In case of an infection (like in the Periamygdaloid abscess, Ludwing angina, etc.) broad-spectrum antibiotics will be used based on the results obtained from the cultures.
The care of the wounds, cures, drainages is predominantly based on the protocol and the indications established by each surgical team and always based on the technique used and the characteristcis of the cases. It is important to know the situation of the drainages and the possible rhythms and debit qualities considered normal. For this reason, it is essential for the surgeon to have all the information available following the patient’s admission.
Like we said at the beginning, tumor surgeries are the predominant type of this surgical procedure. Because of the procedure per se or due to the administration of neoadjuvant therapy with radiotherapy or chemotherapy patients may experience important side effects like impossibility to talk or speech disorders, problems swallowing and altered breathing mechanics; we should not forget here the psychological side of it since many patients will suffer from depression and/or anxiety.30 This impacts quality of life significantly. Rehabilitation is another part of the treatment of these patients, as a matter of fact, it is one of the main parts after the acute phase. The exercises recommended by the physical therapists improve functional capabilities and, eventually, quality of life.31
ConclusionsIn the postoperative care of patients undergoing ENT and NHS surgeries there are processes that due to their potential—though rare—anatomical and functional complications (airway, bleeding, grafts, etc.) require close monitorization in a special unit. The type of unit will depend on the logistics of each particular center. Multidisciplinary approach is essential for a good postoperative recovery as well as to standardize the protocols, not only right after the procedure but also prior to it and during the surgical act too. The technological advances made with robotic techniques has revolutioned this surgical field with promising results; even so, postoperative ICU care within the first few hours of these patients is a routine practice. Due to the qualifications of healthcare providers in the management of serious medical and surgical complications, they are capable of handling these postoperative care periods effective and efficiency.
Conflicts of interestNone reported.
We wish to thank the Oral and Maxillofacial Surgery Department, the ENT Department, and the entire ICU of the Hospital HLA Universitario Moncloa for their cooperation, availability, and collaboration while conducting this paper.
Please cite this article as: Alcázar Sánchez-Elvira L, Bacian Martínez S, del Toro Gil L, Gómez Tello G. Manejo postoperatorio en UCI de cirugía de cabeza y cuello. Med Intensiva. 2020;44:46–53.