Life-sustaining treatment limitation (LSTL) is an increasingly common practice. However, its application is sometimes not clearly reflected in the clinical record–this giving rise to the adoption of measures that could have been avoided, including admission to the intensive care unit (ICU), with the suffering and economical costs this implies. One way to trace patients subjected to LSTL is through an electronic registry allowing identification at all times of these individuals, and of the therapies that have been restricted in each case. The Ethics Committee of our center has developed a tool allowing the identification of patients subjected to LSTL and of the level of intervention required, and offering the association of a patient comfort management protocol.
La limitación de las terapias de soporte vital (LTSV) es una práctica cada vez más extendida, sin embargo, su establecimiento, en ocasiones, no queda claramente reflejado en la historia clínica lo que conlleva la aplicación de medidas que podrían haberse evitado, entre ellas, el ingreso en la unidad de cuidados intensivos (UCI), con el sufrimiento y coste económico que ello origina. Una forma de identificar aquellos pacientes con LTSV es a través de un registro electrónico que permita visualizar en todo momento quiénes son estos enfermos y qué terapias se han restringido. Desde el Comité de Ética de nuestro centro se ha desarrollado una herramienta que nos permite dicha identificación, conocer qué nivel de actuación requiere y asociar un protocolo de tratamiento de bienestar.
The advances of modern Medicine in the treatment and diagnosis of disease have prolonged patient life expectancy. However, in some cases medical effort may only serve to prolong the process of death, with intense suffering for the patients and their relatives, and with an important use of healthcare resources. For this reason, life-sustaining treatment limitation (LSTL) is an increasingly common practice in Departments of Intensive Care Medicine (6.6% in Spain,1 11% in France,2 9.9% in Great Britain,3 10–9.8% in a European Union study,4 and 9.6% in Lebanon5), and results in an important percentage of the registered deaths (30–90%).3,5–10
The forms of LSTL in Intensive Care comprise the limitation of admission to the Intensive Care Unit (ICU), limitation of the start of certain life support measures, or the withdrawal of such measures once introduced.11 Studies carried out in southern Europe reveal differences in the application of these measures, compared with other European regions and the United States.6,7,12,13
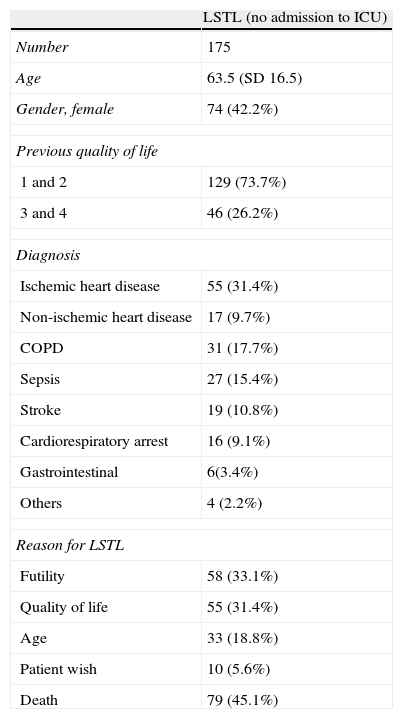
Most published studies consider the suspension of already established life support measures, or the non-introduction of such measures, as forms of LSTL, though few regard non-admission to the ICU as an example of LSTL. In our Department of Intensive Care Medicine, and over a period of two years, we registered all those patients (n=175) who after due evaluation were not admitted to the ICU. Their characteristics are shown in Table 1.
Characteristics of the patients with life-sustaining treatment limitation (LSTL) (“non-admission to the Intensive Care Unit”).
| LSTL (no admission to ICU) | |
| Number | 175 |
| Age | 63.5 (SD 16.5) |
| Gender, female | 74 (42.2%) |
| Previous quality of life | |
| 1 and 2 | 129 (73.7%) |
| 3 and 4 | 46 (26.2%) |
| Diagnosis | |
| Ischemic heart disease | 55 (31.4%) |
| Non-ischemic heart disease | 17 (9.7%) |
| COPD | 31 (17.7%) |
| Sepsis | 27 (15.4%) |
| Stroke | 19 (10.8%) |
| Cardiorespiratory arrest | 16 (9.1%) |
| Gastrointestinal | 6(3.4%) |
| Others | 4 (2.2%) |
| Reason for LSTL | |
| Futility | 58 (33.1%) |
| Quality of life | 55 (31.4%) |
| Age | 33 (18.8%) |
| Patient wish | 10 (5.6%) |
| Death | 79 (45.1%) |
The evaluation of these cases required an average of 45min per patient.
We believe that there is an appreciable number of patients who following admission to the ICU have been included in the LSTL protocol, but who would not have been admitted to our Unit if their antecedents, quality of life, personal wish or will, etc. had been known. We do not consider this to represent incorrect practice (there is always time for withdrawing already initiated life support, directing management towards patient wellbeing), though if admission had been avoided, we could have saved unnecessary suffering for the patients and their families, as well as an important amount of human and economical resources. It is therefore essential to promote patient will documents and LSTL forms.11
In view of the above, and from the Ethics Committee of our center, we considered it advisable to develop an instrument in the patient electronic clinical record allowing us to:
- -
Quickly identify those patients that have been evaluated by the physicians who routinely monitor and know the clinical history as being non-candidates for life support measures.
- -
Determine three levels of intervention, since not all patients require limitation of all available therapeutic measures.
- -
Associate a patient wellbeing protocol and special nursing care plan.
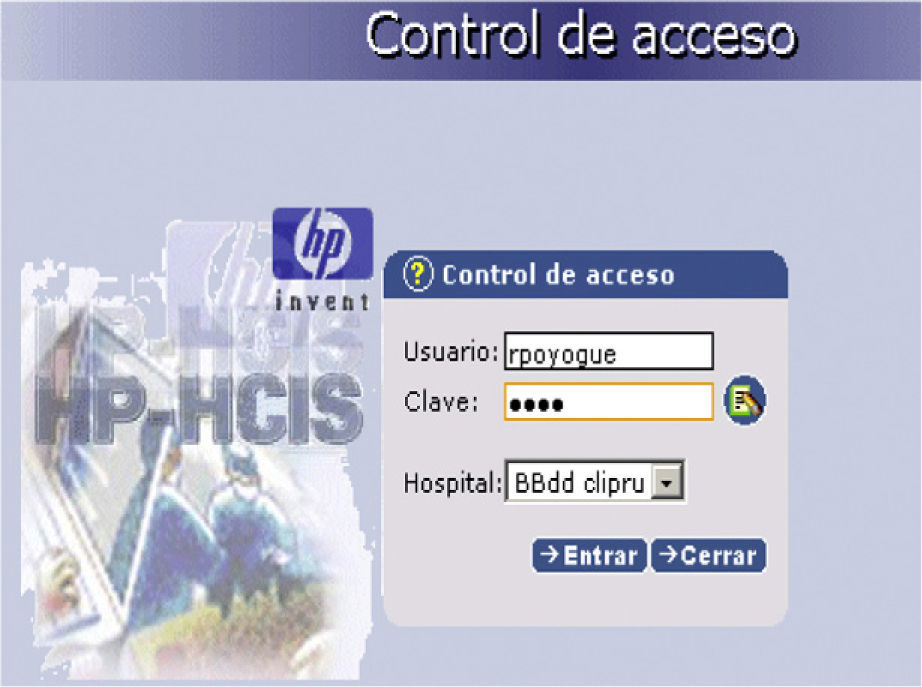
This tool consists of a STOP icon on the main patient page. Activating this option leads to an access or login control requesting the physician code (nursing personnel have read-only access) (Fig. 1).
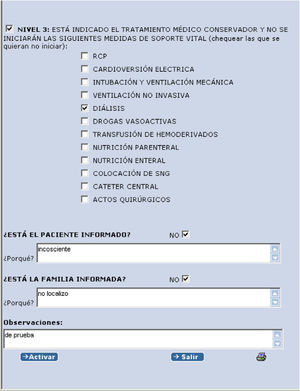
Once the code has been entered, a drop menu appears with three levels of intervention referred to the limitation of care, with a question asking from whom the information has been received. In order to advance through the program, it is necessary to establish a level and answer these questions. In the event of a negative reply, a text field appears requiring an explanation as to why no information has been received. Another text field is reserved for observations and/or comments (Fig. 2).
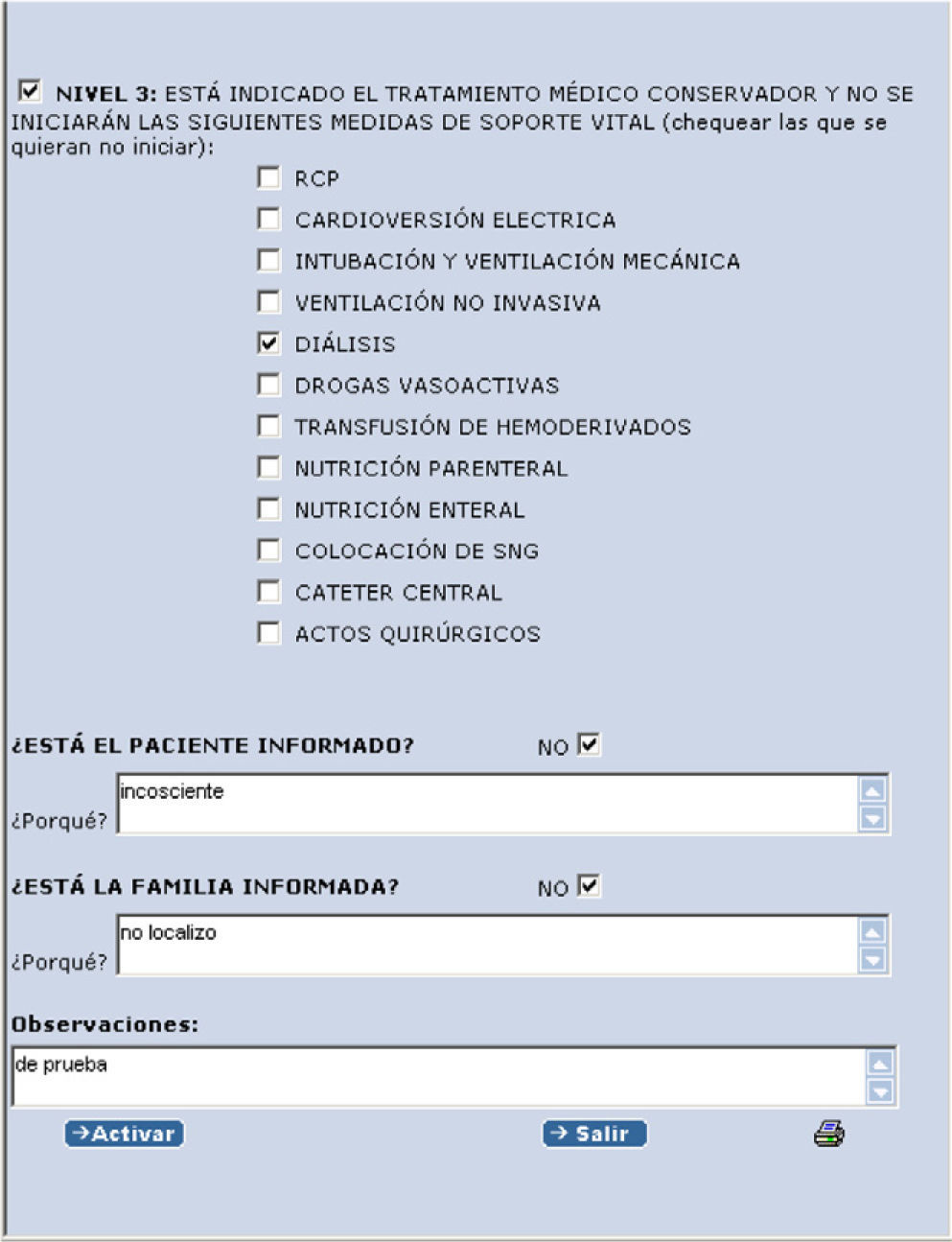
If level 3 is activated, another drop menu appears with different life support measures (Fig. 3).
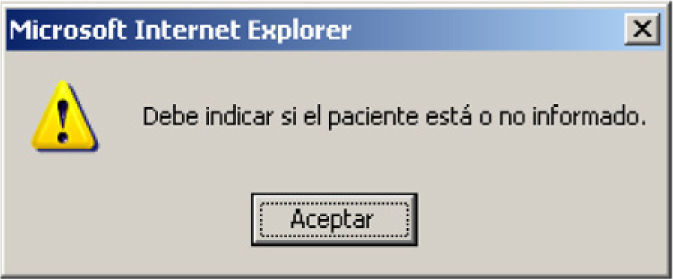
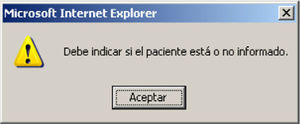
Alerts are generated if the questions referred to the information have not been answered, or if a level has not been established. These problems must be resolved in order to further advance through the program (Fig. 4).
On activating LSTL, the corresponding text is produced and added to the patient clinical record as an annotation (evolutive comment) to the episode in course:
“Dr. Rosa Poyo-Guerrero Lahoz established LSTL level 3 at 11:37:26 on 2011-01-24: conservative medical care is indicated, and the following life support measures will not be started: cardiopulmonary resuscitation (CPR) and dialysis. Patient not informed (under sedoanalgesia). Family informed.”
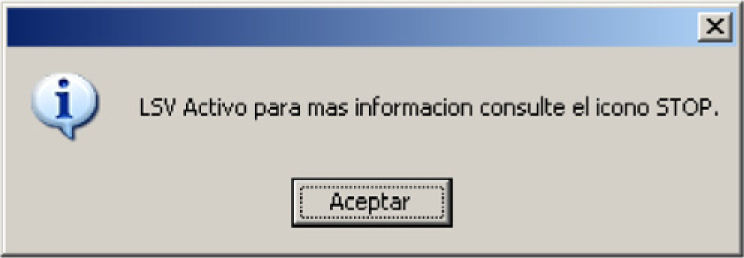
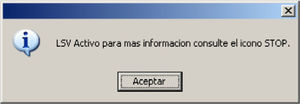
Once LSTL has been established, the following message appears, informing and reminding us of the LSTL status, whenever the patient clinical record is consulted (Fig. 5).
The same physician or any other physician (after due code-based identification) can deactivate LSTL for one reason or other. Observations or comments can be added in the deactivation procedure, and the corresponding annotation likewise will be added to the clinical history (Fig. 6).
If the patient has not been admitted and LSTL is established from the hospital wards, a LIFE SUPPORT LIMITATION problem of increased importance is generated, with the addition of annotations (Fig. 7).
For the nursing personnelWith the STOP icon the nursing personnel can only consult LSTL, and the corresponding treatment and care sheets contain the same icon with an associated special care plan.
Once level 1 has been established, and if considered opportune by the supervising physician, a sedoanalgesia protocol can be activated, with direct indication in the treatment sheet of the prescription of morphic chloride and midazolam boluses, together with morphic chloride in continuous infusion, with doses adjusted to the terminal patient age, weight and needs.
With these measures we believe it is possible to:
- -
Avoid futile treatments, thereby lessening the suffering for the patients and their families, and the associated economical costs.
- -
Provide pain relief and improvement of other symptoms in the end stages of life, based on treatments aimed at affording wellbeing and which can be started easily and quickly.
- -
Obtain a clear and concise registry of the LSTL decisions in the clinical records.
- -
Establish a reminder of patient inclusion in the LSTL protocol for re-evaluation and follow-up.
We developed this tool during a period of one year, after which it was presented in a general session on 12 February 2011, after being accepted by both the Ethics Committee and the Management Board of our center. Application started 24h later, and after three months the number of patients with activated LSTL status reached 120 (3 patients/day). Of these, 54.5% were males, and the median age was 82 years. The Departments from which LSTL activation originated were Internal Medicine (43.8% of the cases), Oncology (14%), Emergencies (13.2%), ICU (12.4%), Cardiology (4%), Hematology, Pneumology and Neurology (2.5%), Traumatology (1.7%), and General Surgery, Pediatrics, Anesthesiology and Nephrology (0.8%).
The reasons or considerations for establishing LSTL were:
Quality of life (24%)
Life expectancy<6 months/terminal (21.5%)
Previous functional limitation (19%)
Futility (20.7%)
Age (8.3%)
Pre-expressed wish (4.1%)
The levels activated were level 1 (14% of the cases), level 2 (43%) and level 3 (43%). In the latter, the restricted life support measures were:
- 1.
Cardiopulmonary resuscitation: 40%
- 2.
Electrical cardioversion: 29%
- 3.
Orotracheal intubation and mechanical ventilation: 40%
- 4.
Noninvasive ventilation: 0%
- 5.
Dialysis: 27%
- 6.
Vasoactive drugs: 27%
- 7.
Blood product transfusions: 0%
- 8.
Parenteral nutrition (TPN): 9%
- 9.
Enteral nutrition: 11%
- 10.
Nasogastric tube (NGT): 7%
- 11.
Central catheter: 12%
- 12.
Surgical interventions: 27%
A change in LSTL level was decided in 10.8% of the cases. The change was from level 1 to level 2 in two cases, from level 2 to level 1 in 9 cases, from level 3 to level 2 in one case, and from level 3 to level 1 in one patient. In no case was LSTL definitively suspended (at discharge). A total of 23.3% of the patients finally presented LSTL level 1, with activation of the available sedoanalgesia protocol in of them.
The family was informed in 93.4% of the cases. In the remaining cases the family either could not be contacted or the patient had no family. The patient was only informed in 18.2% of the cases, since most subjects presented prior dementia, diminished consciousness, etc.
A total of 57 patients (47%) died during admission in which LSTL was activated. Twenty-four patients (20%) were referred to chronic care centers.
In 26% of the patients LSTL was activated in the first 24h after admission. The mean time from hospital admission to LSTL was 6.7 days, while the mean time from LSTL activation to discharge/death was 8.2 days. Six percent of the patients died on the day of LSTL activation. In three cases LSTL was activated from the hospital wards.
Three months after application of the protocol, the generalized opinion among the healthcare professionals was that the tool is useful, as evidenced by its routine use, facilitating the daily work of both the physicians (fundamentally while on duty, when the physician in charge of the patient cannot be consulted) and the nurses–who appreciate knowing the stage in the patient course, the measures to be taken and restricted, and the possibility of implementing a special care plan with which to help and support both the patients and their families.
The main problem reported has been correct differentiation between LSTL levels 2 and 3. Level 3 was initially created to limit only certain life support measures, though without specifying whether the patient could be admitted to the ICU or not. On the other hand, some Departments asked us to expand these measures to include other less aggressive measures, but which cause patient discomfort. We mistakenly decided to incorporate them to level 3, generating confusion in distinguishing patients amenable to conservative medical treatment (level 2) from those at level 2 who in addition to the more aggressive measures also received limitations of other therapies including nasogastric tubes, total parenteral nutrition, etc.
Two modifications are presently pending:
- -
Measures such as nasogastric tubes and central catheters, enteral and parenteral nutrition, and blood product transfusions would move to level 2, as a result of which only the more aggressive measures would remain at level 3.
- -
The LSTL “Admission to the ICU” would be moved to level 3.
We therefore consider that we can and must modify this tool, establishing clear definitions allowing unification of the reasons and levels of LSTL. On the other hand, we consider it necessary to further promote reminders of LSTL with a view to establishing it as a routine practice. In this context, we always must remember that our efforts do not end with LSTL activation, and that multiple measures for patient wellbeing, pain relief, family accompaniment, etc., remain to be applied.
Conflicts of interestThe authors declare no conflicts of interest.
Thanks are due to Dr. F. Gordo, Dr. G. Heras and Dr. Z. Al Nakeeb for their collaboration in this study.
Please cite this article as: Poyo-Guerrero R, et al. Experiencia preliminar en la introducción de la limitación de terapias de soporte vital en la historia clínica electrónica. Med Intensiva. 2012;36:32–6.