Pressure ulcers represent a significant problem for patients, professionals and health systems. Their reported incidence and prevalence are significant worldwide. Their character iatrogenic states that its appearance is preventable and its incidence is an indicator of scientific and technical quality both in primary care and specialized care.
The aim of this review was to identify risk factors associated with the occurrence of pressure ulcers in critically ill patients.
MethodologyThe PRISMA Declaration recommendations have been followed and adapted to studies identifying risk factors. A qualitative systematic review of primary studies has been performed and a search was conducted of the PubMed, The Cochrane Library, Scopus and Web of Science databases. Methodological limitations in observational studies have been considered.
ResultsFrom 200 references, 17 fulfilled the eligibility criteria. These studies included 19,363 patients admitted to intensive care units. Six studies were classified as high quality and 11 were classified as moderate quality. Risk factors that emerged as predictive of pressure ulcers development more frequently included age, length of ICU stay, diabetes, time of MAP <60–70mmHg, mechanical ventilation, length of mechanical ventilation, intermittent haemodialysis or continuous veno-venous haemofiltration therapy, vasopressor support, sedation and turning.
ConclusionsThere is no single factors which can explain the occurrence of pressure ulcers. Rather, it is an interplay of factors that increase the probability of its development.
Las úlceras por presión representan un significativo problema para pacientes, profesionales y sistemas sanitarios. Presentan una incidencia y una prevalencia importantes a nivel mundial. Su carácter iatrogénico plantea que su aparición es evitable y su incidencia es un indicador de calidad científico-técnica tanto en el ámbito de la atención primaria como en el de la especializada.
El objetivo de esta revisión ha sido identificar los factores de riesgo relacionados con la aparición de úlceras por presión en pacientes críticos.
MetodologíaSe siguieron las recomendaciones de la declaración PRISMA adaptadas a la identificación de estudios sobre factores de riesgo. Se ha realizado una revisión sistemática cualitativa de estudios primarios a través de una búsqueda en Pubmed, The Cochrane Library, Scopus y Web of Science. Se consideraron las limitaciones metodológicas en estudios observacionales.
ResultadosDe 200 referencias bibliográficas, 17 cumplieron nuestros criterios de selección. Estos estudios incluyeron 19.363 pacientes ingresados en unidades de cuidados intensivos. Seis se clasificaron como de calidad fuerte y 11 de calidad moderada. Los factores de riesgo que aparecieron más frecuentemente asociados al desarrollo de úlceras por presión incluyeron: edad, tiempo de estancia en UCI, diabetes, tiempo de PAM <60–70mmHg, ventilación mecánica, duración de la ventilación mecánica, terapia de hemofiltración venovenosa continua o diálisis intermitente, tratamiento con drogas vasoactivas, con sedantes y cambios posturales.
ConclusionesNo aparecen factores de riesgo que por sí mismos puedan predecir la aparición de la úlcera por presión. Más bien se trata de una interrelación de factores que incrementan la probabilidad de su desarrollo.
Pressure ulcers (PUs) have a significant impact upon patient morbidity–mortality and quality of life, and are a cause of concern for both the patients and their families, as well as for health professionals and healthcare systems. Pressure ulcers are commonly found at any healthcare level, particularly in patients with mobility problems and advanced age.1 Although the development of PUs is not intrinsically regarded as a cause of mortality during hospital admission, such lesions are associated to mortality and to other complications in the course of patient recovery: they increase the risk of infection and of in-hospital malnutrition, prolong hospital stay, increase the nursing care burden, and result in greater healthcare costs.2
Many incidence and prevalence studies have brought the problem into focus, but have been based on different indicator calculation methods, as well as on different inclusion and exclusion criteria. Patients admitted to the Intensive Care Unit (ICU) are at a high risk of developing PUs, with an incidence of between 3.3% and 52.9%.3,4 Such patients generally do not notice the increased tissue pressure or fail to react to it adequately because of sedation, analgesia and/or the use of muscle relaxants. Furthermore, the background disease and hemodynamic instability increase the risk of PUs.
Despite the magnitude of this healthcare problem, few studies have quantified the direct association between risk factors and the appearance of PUs, and some of the published articles are fundamented upon assumptions of a general nature.5 At present, ICUs use instruments for assessing the risk of PUs that have not been specifically developed for this care setting and therefore might not be adequate, since they do not take into account risk factors that are practically exclusive of such Units.6
The importance of the different aspects implicated in the appearance of PUs in critical patients is the subject of permanent controversy.5,7 It is therefore particularly important to examine the direct relationship between the risk factors and the appearance of PUs in these patients, with a view to establishing specific interventional measures. Although there are aspects upon which no direct or effective impact can be made, in some cases interventions targeted to a single element can modify the effects of the rest of the implicated factors.8
The aim of this systematic review is to identify the risk factors related to the appearance of PUs in critical patients admitted to the ICU.
Material and methodsA systematic review of primary studies has been carried out. The PRISMA Declaration was followed, and the review protocol was defined prior to data collection, with the purpose of reducing the impact of bias inherent to the authors and to promote transparency regarding the methods and the process.9 For the evaluation of methodological quality, we used the Critical Appraisal Skills Program España (CASPe) templates10,11 according to the type of study, with the aim of assessing the risks referred to screening, measurement, withdrawal or classification bias, as well as to confounding factors, outcome bias and other sources of bias. We selected those studies yielding a score of over 6. The studies with a score of between 6 and 8 were regarded as being of moderate quality, while those of over 8 points were taken to be of high quality.
A meta-analysis of the data was not possible, due to the heterogeneity of the designs, risk factors studied and results obtained. Since the primary objective was to identify these factors rather than to quantify the effect size of correlation to the development of PUs, we conducted a narrative synthesis in which the risk factors were categorized into domains and sub-domains, with identification of those included in the multivariate model and which were found to be statistically significant (p<0.05).
Type of studiesInclusion criteria: Primary studies published between 1 January 2008 and 1 August 2016, involving patients over 18 years of age in the ICU setting, and with the development of PUs as outcome variable; prospective or retrospective observational studies or clinical trials conducting multivariate analysis for the identification of risk factors capable of influencing the appearance of ulcers, and with moderate to high methodological quality.
Exclusion criteria: Studies involving pediatric populations, studies with cross-sectional designs, publications in languages other than English, Spanish or Portuguese, studies in animals, and studies in which over 20% of the sample was excluded from the analysis due to dropout, death, loss to follow-up or missing data.
Interventions and outcomesThe evaluated outcome was the appearance of PUs during admission to the ICU, categorized into stages I–IV, independently of whether the original studies considered the development of PUs in stages ≥I or in stages ≥II as outcome variable.
The interventions were the risk factors identified in the articles and which were included in the multivariate analyses.
Search strategyA search was made of the PubMed, Cochrane Library, Scopus and Web of Science (WOS) databases using the descriptors “intensive care units”, “pressure ulcer” and “risk factors”, combined with the operator “and”. All were entered as in the MeSH thesaurus. We excluded the pediatric population, including newborn infants. The complete search strategy in WOS is shown below:
Topic: (“intensive care units”) and Topic: (“pressure ulcer”) and Topic: (“risk factors”) not Topic: (child*) not Topic: (neonat*).
Restrictions: Language: (English or Portuguese or Spanish) and Areas of investigation: (“critical care medicine”).
Data extraction and analysisThe titles and abstracts were screened by two independent reviewers and were checked by a third reviewer. The potentially relevant abstracts were retrieved in full text format and were evaluated by a reviewer with the aim of selecting those studies that met the specified inclusion criteria. They were also checked by a second reviewer. Agreement between the reviewers was assessed based on the Cohen kappa statistic, which was found to be 0.79 (95% confidence interval [95%CI]: 0.47–1.10), indicating good agreement. We conducted a statistical review in those cases where the statistical analyses were not clearly stated in the document, and where inclusion of the study was therefore uncertain. Any disagreement was resolved by consensus among the group of reviewers. Once the studies meeting the inclusion criteria were selected, the data were extracted by a single reviewer and checked by a second reviewer. Information was compiled in relation to the study design, mediating variables (risk factors) and measurement instruments used, possible confounding factors and the methods used to control them, and statistical analysis—identifying the risk factors found to be significant and quantifying the effect size of correlation to the development of PUs.
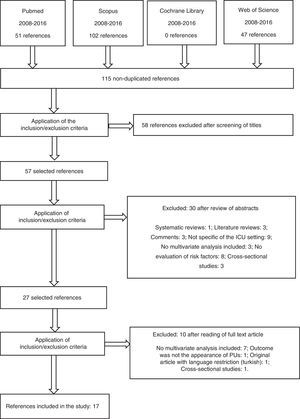
ResultsA total of 200 literature references were identified: 51 in PubMed, 102 in Scopus and 47 in WOS. After the review process, a total of 17 articles were found to meet the study inclusion criteria (Fig. 1).
Characteristics of the studiesA total of 19,363 patients were included. The mean incidence of PUs was 18.31% (range 3.3–39.3%). A total of 1460 patients developed at least one PU during admission to the ICU. Five studies regarded the development of PU in stages ≥II as outcome variable.3,5,7,12,13 The other studies regarded stages ≥I as outcome variable. The rest of the characteristics are described in Appendix B (Table 1).
One publication was a case–control study,14 and four were retrospective registry review-based cohort studies.3,12,15,16 The rest of the publications were prospective cohort studies. Two studies3,8 performed survival analysis (Cox proportional hazards model), while the rest resorted to logistic regression models.
All the studies considered a dichotomic outcome referred to the appearance of PUs, i.e., development of PU or no development of PU. The 17 studies produced multivariate models designed to identify those risk factors which proved statistically significant in predicting the appearance of PUs (independently or adjusted for the rest of the factors). The articles evaluated an average of 11 potential risk factors in the multivariate analysis (range 4–15). The adjusted models identified an average of four predictive factors (range 2–10).
Risk factors: domains and subdomainsAppendix B (Table 2) summarizes the risk factors (significant and nonsignificant) in the multivariate models, classified by domains and subdomains.
Domain 1: demographic characteristicsSubdomain age. All the studies included age as a possible risk factor. In 6 of them5,12,15,17–19 the development of PU was significantly correlated to older age in the multivariate analysis. In one study19 age was regarded as a risk factor if the patient was ≥70 years old (odds ratio [OR][95%CI]=2.14 [1.27–3.62]).
Subdomain gender. Two studies identified gender as being significant in the multivariate analysis. In one of them,20 of moderate methodological quality, the male gender was identified as a protective factor (OR [95%CI]=0.15 [0.03–0.71]). In contrast, the other study,21 of high methodological quality, found males to be 5 times more likely to develop PUs (OR [95%CI]=5.60 [1.42–22.09]).
Subdomain BMI. The body mass index (BMI) was identified as a risk factor in the univariate analysis of 5 articles3,8,12,14,18 and in an adjusted model,12 with BMI <18.5kg/m2 being associated to the development of PUs (OR [95%CI]=2.70 [1.45–5.04]).
Domain 2: time factorTwelve studies (70%)3,5,8,13–18,21–23 identified length of stay in the ICU as a risk factor. In 7 of them13,15,17,18,21–23 the result was significant and was entered in the adjusted model. In almost all of the studies the odds ratio was very close to 1. In the case of Tayyib et al.17 the odds ratio was 1.83 (1.01–3.30).
Only one study included the season of the year as a possible risk factor5 – the winter months being identified as the season significantly associated to the development of PUs in the multivariate analysis (OR [95%CI]=4.60 [1.99–10.59]).
One study3 found patients undergoing surgery in the first 24h of admission to have a significantly lesser risk of developing PUs, though variation was observed over time: patients hospitalized for over 5 days after surgery had a discretely greater risk of developing PUs. The hazard ratio (HR) (95%CI) was 0.21 (0.14–0.31) during the first 5 days, and 0.25 (0.19–0.33) after this time.
Domain 3: type of admissionOne study24 established a correlation to the type of admission (from the emergency room, scheduled surgery operating room or hospital ward). Patients coming from the operating room after scheduled (elective) surgery developed fewer PUs than those coming from the emergency room (OR [95%CI]=0.041 [0.004–0.470]). In another investigation,17 admission from the emergency room was positively associated to the development of PUs in the univariate analysis. In contrast, another study7 found no differences between patients coming from the emergency room and those admitted after scheduled surgery. Other authors distinguished between medical and surgical admissions,5,8,14,18 and one study5 associated surgical admission to the development of PUs only in the univariate analysis.
Subdomain trauma. Of the 7 studies5,8,13,14,17,18,23 that examined the association between admission to the ICU due to trauma and the appearance of PUs, only one18—of moderate methodological quality—observed a correlation in the multivariate analysis (OR [95%CI]=15.95 [3.72–68.65]). In the remaining 6 studies – two of which were of high methodological quality8,14—no such significant association was observed in the univariate analysis.
Subdomain surgery. The study published by O’Brien et al.,12 of moderate methodological quality, explored the possible intraoperative risk factors that could contribute to the development of PUs. Non-cardiac surgery was found to be significant in the multivariate analysis (OR [95%CI]=1.84 (1.31–2.59]).
Domain 4: comorbiditiesSubdomain smoking. Three studies evaluated smoking as a possible risk factor.12,14,18 One of them,18 of moderate methodological quality, reported significance in the multivariate analysis (OR [95%CI]=1.03 [1.01–1.06]).
Subdomain diabetes. Eight studies evaluated diabetes as a possible risk factor.3,12,14–19 The multivariate analyses of Nassaji et al.18 and Slowikowski and Funk19 identified diabetes as being significantly associated to the appearance of PUs (OR [95%CI]=5.58 [1.83–18.70] and OR [95%CI]=1.93 [1.11–3.35], respectively). Another study12 observed significance for this parameter in the univariate analysis.
Subdomain previous vascular disease. Previous vascular disease was found to be a significant risk factor in one study,7 of high methodological quality. In a first phase the authors examined the risk factors 24h before the appearance of PUs, while in a second phase they did so 48h before the appearance of PUs (OR [95%CI]=4.51 [1.99–10.24] and OR [95%CI]=2.85 [1.29–6.30], respectively).
Subdomain renal failure. Two studies evaluated renal failure as a possible risk factor.12,19 Only the study of O’Brien et al.,12 of moderate methodological quality, found renal failure to be significantly associated to the appearance of PUs in the multivariate analysis (OR [95%CI]=1.75 [1.27–2.39]).
Domain 5: diagnosis upon admissionSubdomain sepsis. Five studies evaluated sepsis or septic shock upon admission as a possible risk factor.5,13,14,16,17 The study published by Yepes et al.13considered whether the patient presented infection upon admission as evidenced by positive cultures or infection-related diagnoses, and in this regard sepsis proved significant in the multivariate analysis (OR [95%CI]=2.89 [1.16–7.22]). The rest of the studies did not report statistical significance for sepsis in the univariate analysis.
Domain 6: vital signsSubdomain duration of mean blood pressure (MBP) <60–70mmHg. Two studies16,23 identified this parameter as a risk factor in the multivariate analysis. One of them,16 of moderate methodological quality, examined the duration of hypotension refractory to vasopressive drug treatment, defined as MBP <60mmHg (OR [95%CI]=1.09 [1.02–1.17]), while the other study23 observed whether the patients presented MBP <70mmHg once in the course of each shift. The percentage of such observations was regarded as a risk factor (OR [95%CI]=1.02 [1.01–1.03]).
Subdomain body temperature ≥38.5°C. In the first phase of the study published by Nijs et al.,7 of high methodological quality, such temperature elevations were found to be negatively associated to the development of PUs (OR [95%CI]=0.18 [0.18–0.92]). The mechanism underlying the protective effect of a temperature of ≥38.5°C is not clear, though according to the authors, it could be a consequence of the vasodilatation caused by hyperthermia, with improved oxygenation secondary to the increase in blood flow.
Domain 7: fecal incontinenceFecal incontinence was associated to the appearance of PUs in one study18 (OR [95%CI]=3.42 [1.45–8.06]), while another investigation8 did not find it to be significant in the univariate analysis.
Domain 8: medication administeredSubdomain treatment with amines. Ten studies considered treatment with amines,7,8,12–16,19,22,23 and in four of them the parameter was entered in the adjusted model.7,8,16,23 In the first phase of the study of Nijs et al.,7 treatment with dopamine ≤5μg/kg/min showed OR (95%CI)=6.05 (1.88–19.54). Another study8 analyzed the administration of noradrenalin as a qualitative variable, without taking into account the dose or duration of treatment (OR [95%CI]=3.68 [1.12–12.06]). In the case of Cox and Roche,16 vasopressin was found to be the only vasoconstrictor with significance in their adjusted model (OR [95%CI]=4.81 [1.66–13.92]). Lastly, in the investigation of Llaurado-Serra et al.,23 a significant association was observed between treatment with vasoactive drugs and the development of PUs (OR [95%CI]=1.02 [1.02–1.03]).
Subdomain sedation. Six studies evaluated sedation as a possible risk factor.7,8,13,19,23,24 In four publications7,13,19,24 it was regarded as a dichotomic parameter. Three found it to be significant in the multivariate analysis. Nijs et al.7 identified sedation as a protective factor in both phases of their study (OR [95%CI]=0.30 [0.13–0.70] and OR [95%CI]=0.27 [0.11–0.65], respectively). In the case of Roca-Biosca et al.,8 the study variable was the days of sedation, which was likewise identified as a protective factor (OR [95%CI]=0.90 [0.83–0.99]). Llaurado-Serra et al.23 found a significant relation between sedation treatment and the development of PUs (OR [95%CI]=1.02 [1.01–1.03]).
Domain 9: devicesSubdomain mechanical ventilation (MV). Mechanical ventilation was considered as a dichotomic variable in three studies.7,17,19 Five publications evaluated the duration of MV as a possible risk factor,5,13,14,16,23 and four of them found it to be significant in the multivariate models.5,14,16,23 Specifically, in the study of Cox and Roche,16 patients requiring MV for more than 72h had a 23-fold greater probability of developing PUs (OR [95%CI]=23.60 [6.42–86.66]).
Another investigation12 evaluating the presence of an artificial airway as possible risk factor observed statistical significance, with an OR (95%CI) of 5.28 (3.63–7.67).
Subdomain extrarenal filtration or intermittent dialysis. Four studies considered this possible risk factor as a dichotomic variable,7,8,14,19 and two of them found it to be significantly associated to the development of PUs.7,14 In the two phases of the study of Nijs et al.,7 intermittent dialysis or continuous veno-venous hemofiltration was associated to the development of PUs (OR [95%CI]=3.77 (1.03–13.86) and OR [95%CI]=9.43 [3.01–29.51], respectively). In the case of Catalá et al.,14 the OR (95%CI) was 3.55 (1.31–9.64).
Domain 10: complicationsSubdomain acute respiratory failure. Acute respiratory failure was identified as a risk factor in only one multivariate model3 (HR [95%CI]=2.68 [2.16–3.33]). In another three studies5,13,15 it was not found to be significant in the univariate analysis.
Subdomain infection, septic shock. Infection was identified as a possible risk factor in one study,15 with the observation of significance in the univariate analysis. The same occurred with septic shock in the study published by Manzano et al.5
Domain 11: hematological parametersSubdomain albumin. One publication20 considered the mean albumin level at the time of admission to the ICU and the daily levels up until the development of PUs. The patients who developed PUs had lower serum albumin levels (OR [95%CI]=11.62 [1.92–70.4]). In another investigation23 increased albumin levels were identified as a protective factor (OR [95%CI]=0.62 [0.39–0.98]).
Subdomain hemoglobin. Anemia was significantly associated to development of PUs in one study (OR [95%CI]=2.68 [1.22–5.91]).18 In other studies7,20 hemoglobin concentration was entered in the multivariate analysis, though no association was established.
Domain 12: preventive measuresSubdomain dynamic surface. One study8 found the days of dynamic surface device utilization to exert a significant protective effect against the appearance of PUs (OR [95%CI]=0.87 [0.81–0.94]).
In the two phases of the study published by Nijs et al.,7 the multivariate analysis paradoxically identified a positive association between the use of alternating pressure cushions and the development of PUs.
Subdomain postural changes. Four studies evaluated postural changes as an independent variable.7,8,17,24 One of them24 included postural changes every 2h (though with more frequent application if required by the patient), and patient repositioning. The univariate analysis, based on the daily frequency of postural changes, found no association between the frequency of postural changes and repositioning and the development of PUs. However, in the multivariate analysis, the patients that developed PUs received significantly fewer postural changes (OR [95%CI]=0.45 [0.21–0.97]).
In another study,17 less frequent postural changes were positively associated to the development of PUs (OR [95%CI]=250.04 [5.23–11,954.16] in PUs of all stages and OR [95%CI]=2.96 [1.23–7.15] in stages ≥II). However, in another investigation8 the frequency of postural changes in days only showed significance in the univariate analysis.
In the case of Nijs et al.,7 a frequency of the postural changes of ≥6/day was found to be significantly associated to the development of PUs in both the first phase of the study and in the second phase (OR [95%CI]=30.21 [12.20–74.77] and OR [95%CI]=3.63 [1.09–12.05], respectively).
Subdomain sitting position. A negative correlation between the sitting position and the development of PUs was observed in the first phase of the study published by Nijs et al.7 (OR [95%CI]=0.08 [0.02–0.27]) – this being the only identified protective measure.
DiscussionThis systematic review shows the incidence of PUs in the ICU to be within the range 3.3–39.3%. The 17 studies included in the review evaluated an average of four risk factors for the development of PUs. The risk factors most frequently associated to the development of PUs were patient age, the length of stay in the ICU, diabetes, the duration of MBP <60–70mmHg, mechanical ventilation, continuous veno-venous hemofiltration or intermittent dialysis, vasoactive and sedative drug treatments, and postural changes.
The risk of PUs increases with age due to a number of reasons, such as reduced activity or mobility, diminished tissue tolerance and pain perception, or an increased presence of comorbidities.18,25 In 6 of the studies of our review (35.3%), patient age was identified as a risk factor for the appearance of PUs. Patients over 60 years of age were at greater risk. This evidence points to the need to incorporate patient age as a risk factor in the PU risk assessment scales.26,27
With the exception of one study18 (in which one of the inclusion criteria was the male sex), the different publications evaluated patient gender as a risk factor, with the identification of a statistically significant association in three univariate analyses20,21,24 and finally in two multivariate analyses.20,21 However, Ülker and Yapucu20 underscored that the data referred to gender must be interpreted with caution. In their study, gender as such did not pose a real risk, since the women were older than the men, and this could have caused the female population to be more vulnerable. In sum, there is little evidence to suggest that gender is a factor associated to the development of PUs.
The evidence is limited and controversial regarding the association between BMI and the development of PUs—correlations in some cases being reported with low BMI values, and with high BMI values or obesity in others. As an example, in the study published by O’Brien et al.,12 low weight was associated to the development of PUs in the multivariate analysis, while Tescher et al.3 found that patients with BMI <18kg/m2 had a 1.65-fold greater risk of developing PUs on the following day compared with patients presenting BMI 18–25kg/m2 (only in the univariate analysis). In contrast, in other studies,8,18 greater BMI was identified as a risk factor in the univariate analysis. Lastly, Catalá et al.14 considered BMI ≥40kg/m2 to be a confounding factor, since patients with morbid obesity required more days of mechanical ventilation.
In relation to the time factor, the longest stays in the ICU corresponded to the studies of Cremasco et al.21 and Yepes et al.,13 who recorded a greater incidence of PUs than in the studies of Sayar et al.22 and Cox,15 with shorter stays. In the study carried out by Cremasco et al.,21 the mean stay among the patients that developed PUs was 23.39 days, versus 9.40 days among those who did not develop ulcers. In the study of Yepes et al.,13 the mean stay among the patients that developed PUs was 21.18 days, versus 8.58 days among those who did not develop ulcers. In contrast, in the case of Sayar et al.,22 the mean stay among the patients that developed PUs was 14.05 days, versus 4.66 days among those who did not develop ulcers, and in the study published by Cox,15 the mean stay among the patients that developed PUs was 11.7 days, versus 3.3 days among those who did not develop ulcers. According to this latter study, 66% of the patients developed PUs within the first 6 days of stay.
With regard to the type of admission, and although little evidence is available, those patients admitted from the emergency room had a greater risk of developing PUs.17,24 The studies carried out to date do not allow us to establish whether medical or surgical admission can be regarded as a risk factor for the development of PUs. Admission of trauma patients as a possible risk factor yielded heterogeneous results, though most studies reported no correlation to the development of PUs.
In relation to vasoactive medication and sedation, the studies were heterogeneous in terms of the variables considered. Further studies are needed to define the relationship between the development of PUs and the type of vasopressor, its dosage and the duration of treatment. In some cases, two or three vasopressors are administered, and this could increase the risk of developing PUs.28 In the case of sedation, Nijs et al.7 found sedative use to act as a protective factor – this being consistent with the observations of Roca-Biosca et al.8 referred to the number of days of sedation. In contrast, Llaurado-Serra et al.23 identified sedation as a risk factor. The evidence that some concrete medication predisposes to the development of PUs is therefore limited.
With regard to the subdomain mechanical ventilation, 6 of the 9 studies that analyzed this parameter as a risk factor confirmed its influence in the multivariate analysis. The duration of MV was evaluated in three studies that established the existence of invasive or noninvasive MV as an inclusion criterion.5,14,23 In the case of Manzano et al.,5 MV increased the risk of development of PUs by 4.2% for each day of MV, versus 7.5% for each day of MV in the study of Catalá et al.14 In the study published by Cox and Roche,16 those patients requiring MV during >72h presented a 23-fold greater probability of developing PUs.
In the case of Nijs et al.,7 a frequency of postural changes of ≥6/day and the use of alternating pressure cushions were identified as risk factors for PUs. These results are a cause for concern, since different clinical practice guides recommend precisely these measures for the prevention of PUs. The authors offer two explanations for these results. The first is that the patients at risk were adequately identified, but the preventive measures were applied too late to avoid the development of PUs. The second offered explanation is that the nursing staff only started to apply the preventive measures once the PUs became visible.
A strength of this review is that all the included studies underwent methodological quality assessment in order to identify biases and limitations. A clear limitation is the large number of variables used in the studies to describe the risk factors, which moreover did not coincide among the different publications—thereby precluding use of the data for conducting a meta-analysis. Another limitation refers to the use of different outcome variables: some studies considered PUs in stage ≥I, while others considered PUs in stage ≥II. Some authors suggested that the risk factors associated to PUs in stage I differ from those associated to stage II.29 On the other hand, in relation to the study designs involved, we included prospective and retrospective observational studies, as well as clinical trials, with the final incorporation of four retrospective studies3,12,15,16—a fact that could pose a limitation regarding the quality of the collected data.
ConclusionsIn this systematic review, the risk factors most frequently associated to the development of PUs have been patient age, the length of stay in the ICU, diabetes, the duration of MBP <60–70mmHg, mechanical ventilation, continuous veno-venous hemofiltration or intermittent dialysis, treatment with vasoactive drugs, sedative use, and postural changes.
In most cases, the evidence is limited and does not allow the identification of risk factors intrinsically predictive of the development of PUs. Rather, the interrelation of different factors could increase the probability of ulcer development.
It would be advisable for the scientific societies to encourage definition of the minimum data set to be incorporated to studies on PUs and the risk factors independently associated to the development of such ulcers, given the heterogeneity of the existing studies.
AuthorshipThe contributions of each of the authors in conducting this study are detailed below.
M. Isabel González-Méndez has participated in the definition of the search strategy, the screening of titles and abstracts, the retrieval of relevant articles in full text format, selection of the studies meeting the inclusion criteria, calculation of the kappa coefficient, development of the summarizing table on the characteristics of the studies and the summarizing table on evidence for risk factors, preparation of the first manuscript draft, and approval of the final version of the manuscript for publication.
Francisco Manuel Carrasco-Cebollero has participated in the screening of titles and abstracts, the evaluation of methodological quality, critical manuscript review, and approval of the final version of the manuscript for publication.
Marta Lima-Serrano designed the investigation and the systematic review protocol, and has participated in the definition of the search strategy, review of the selection of the studies meeting the inclusion criteria, checking of the evaluation of methodological quality, checking of the summarizing table on the characteristics of the studies and of the summarizing table on evidence for risk factors, review of the statistical analyses presented by the studies, critical manuscript review, and approval of the final version of the manuscript for publication.
Joaquín Salvador Lima-Rodríguez has participated in the design of the investigation, approval of the review protocol, supervision of correct conduction of the study, preparation of the final manuscript version, and approval of the final version of the manuscript for publication.
Conflict of interestAll the authors declare that they have no financial or personal ties to other people or organizations that could have an inappropriate influence upon their work.
Please cite this article as: Lima Serrano M, González Méndez MI, Carrasco Cebollero FM, Lima Rodríguez JS. Factores de riesgo asociados al desarrollo de úlceras por presión en unidades de cuidados intensivos de adultos: revisión sistemática. Med Intensiva. 2017;41:339–346.