To describe the variables related to effective cough capacity and the state of consciousness measured prior to decannulation and compare their measured values between the different areas of care such as the Intensive Care Unit (ICU), General ward and Mechanical Ventilation Weaning and Rehabilitation Centers (MVWRC). Secondarily analyze the evolution of patients once decannulated.
DesignCase series, longitudinal and prospective.
ScopeMulticentric 31 ICUs (polyvalent) and 5 MVWRC.
PatientsTracheostomized adults prior to decannulation.
MeasurementsMaximum expiratory pressure, peak expiratory flow coughed (PEFC), Glasgow Coma Scale (GCS).
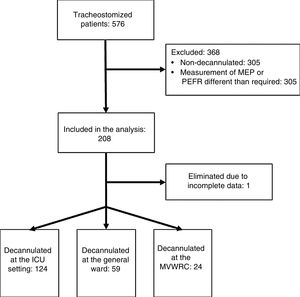
ResultsTwo hundred and seven decannulated patients, 124 (60%) in ICU, 59 (28%) General ward and 24 (12%) in MVWRC. The PEFC presented differences between the patients (ICU 110–190l/min versus MVWRC 167.5–232.5l/min, p<.01). The GCS was different between General ward (9–15) versus ICU (10–15) and MVWRC (12–15); p<.01 and p<.01, respectively. There were differences in the days of hospitalization (p<.01), days with tracheostomy (<0.01) and the number of patients referred at home (p=.02) between the different scenarios.
ConclusionThere are differences in the values of PEFC and GCS observed when decannulating between different areas. A considerable number of patients are decannulated with values of PEFC and maximum expiratory pressure below the suggested cut-off points as predictors of failure in the literature. No patient in our series was decanulated with an GCS<8, this reflects the importance that the treating team gives to the state of consciousness prior to decannulation.
Describir las variables relacionadas con la capacidad tusígena efectiva y el estado de la conciencia medidas previo a la decanulación y comparar sus valores medidos entre los diferentes ámbitos de atención como la Unidad de Cuidados Intensivos (UCI), sala general y centros de desvinculación de la ventilación mecánica y rehabilitación (CDVMR). Secundariamente analizar la evolución de los pacientes una vez decanulados.
DiseñoSerie de casos, longitudinal y prospectiva.
ÁmbitoMulticéntrico 31 UCI (polivalentes) y en 5 CDVMR.
PacientesAdultos traqueostomizados previos a la decanulación.
MedicionesPresión espiratoria máxima, pico flujo espiratorio tosido (PFET), Glasgow Coma Scale (GCS).
ResultadosDoscientos siete pacientes decanulados, 124 (60%) en UCI, 59 (28%) en sala general y 24 (12%) en CDVMR. El PFET presentó diferencias entre los pacientes (UCI 110-190l/min versus CDVMR 167,5-232,5l/min; p<0,01). El GCS fue diferente entre la sala general (9 -15) versus UCI (10-15) y CDVMR (12 - 15); p<0,01 y p<0,01, respectivamente. Hubo diferencias en los días de internación (p<0,01), los días con traqueostomía (<0,01) y la cantidad de pacientes derivados a domicilio (p=0,02) entre los distintos escenarios.
ConclusiónExisten diferencias en los valores medidos de PFET y GCS entre los diferentes ámbitos. Una considerable cantidad de pacientes son decanulados con valores de PFET y presión espiratoria máxima por debajo de los puntos de corte sugeridos como predictores de falla en la literatura. Ningún paciente de nuestra serie fue decanulado con un SCG <8 puntos, esto refleja la importancia que le otorga el equipo tratante al estado de conciencia al momento de la decanulación.
The decision to remove the tracheostomy cannula or the decannulation procedure is influenced by multiple factors. Since it is a complex procedure, it is usually guided by algorithms or protocols that may vary from one center to the next and that establish the criteria to be followed to move ahead with the decannulation procedure.1–7 Although it is true that not all centers develop decannulation protocols, decannulation failure is a rare event and according to the percentages published in the medical literature it is between 0% and 6%.8–12
The criteria that we usually find in most protocols have to do with the capacity to protect the airway with an effective cough, the proper passage of air through the airway, and a proper swallowing function.13–15
The protection of the airway is assessed through the state of consciousness and the capacity to cough.1,4
The state of consciousness is usually recommended as a variable to take into account when making the decision to decannulate a patient. However, there is no consensus on how to assess it and what the minimum state is to be able to predict successful decannulations.8,13,14
When it comes to the capacity to produce cough, the main indicators that are usually evaluated in the process of decannulation are the peak expiratory flow rate (PEFR) and the maximum espiratory pressure (MEP). The relevant clinical values for these predictors are usually cause for controversy since different authors speak of different values.13,14
Another relevant factor is the heterogeneity of the settings where tracheostomized patients can be hospitalized such as intensive care units (ICU), general wards (GW), and mechanical ventilation weaning and rehabilitation centers (MVWRC). In these settings, the process of decannulation is usually started and, at times, completed.16 Although, in these proposed settings, we find different populations and the complexity of healthcare provided is different, the decannulation criteria followed are usually similar or even identical.16–18
To this date there are no studies describing the relevant clinical variables in different settings where the tracheostomized patient has been hospitalized. That is why we wish to describe the different variables associated with the capacity to produce an effective cough and the state of consciousness frequently used in the process of decannulation and compare the values measured at different healthcare settings such as the ICU, the GW and the MVWRC. Also, we wish to study the progression of patients following decannulation.
Materials and methodsOne multicenter study designed as a series of longitudinal prospective cases was conducted including 31 polyvalent ICUs and 5 MVWRCs in Argentina from June 1, 2004 to January 31, 2015.
Prior to recruitment, each center obtained the approvals from each center's teaching and research committee and ethics committee.
The sample was defined by the following eligibility criteria:
Inclusion criteria: patients over 18 years old tracheostomized during their stay at an ICU or GW and who were already tracheostomized by the time they were admitted to the MVWRCs. Also, they should not be on mechanical ventilation and they should be eligible for decannulation (patients with a decision to decannulate by the treating team).
Exclusion criteria: patients who had never been decannulated. Decannulated patients whose data came from centers that do not make MEP and PEFR assessments using the technique recommended by this study.
Elimination criteria: data missing from the three variables of the main outcome. In the case of data missing from one or two variables of the main outcome (GCS, PEFR or MEP), the patient was only excluded for the analysis of the variable with the missing data.
Study variablesPredictive variables: age, sex, Charlson score at admission, associated prior medical history, reason for ICU admission, type of procedure to perform the tracheostomy (percutaneous or surgical).
All patients were followed up to 30 days after decannulation and there were variables during the patient's progression such as lack of decannulation (recannulation within 7 days from decannulation for whatever reason), days on tracheostomy (ever since the tracheostomy was performed and up until the patient was decannulated), total stay (from ICU and/or MVWRC admission and up to meeting one criterion for study completion such as discharge, referral to other centers, or termination of follow-up 30 days after decannulation and 30-day mortality rate).
Independent variables: decannulation setting (ICU, GW, or MVWRC).
Outcome variables: state of consciousness measured using the Glasgow Coma Score (GCS), and cough capacity measured using the PEFR and the MEP.
ProceduresPatients were categorized into different levels of healthcare and depending on the healthcare setting where decannulation actually happened. Three levels were found here: patients decannulated at the ICU, GW, and MVWRC settings.
Given this is an observational pragmatic study, the typical protocols used by the centers for measuring purposes were not asked to change. However, centers were actually urged to describe the methods used to measure the PEFR, the MEP, and the GCS.
In order to make homogeneous and comparable measurements, only patients from centers that had already measured the outcome variables following the procedures described in the Annex 1 (supplementary data associated with this article can be found in the online version) were taken into consideration.
Statistical analysisCategorical variables are expressed as absolute count and percentage. The variables measured using the discrete or continuous numerical scales are expressed as measures of the main trend and dispersion based on the distribution found.
The Kolmogorov–Smirnov test was used to test the goodness-of-it (GOF). The equality of variances was established using Levenne statistical test.
To study the comparisons among the different levels of healthcare, the chi-squared test for nominal variable comparison and the Kruskal–Wallis test for continuous variable comparison among the different levels of healthcare provided were used. In order to identify pairs with significant differences, one post hoc analysis using Dunn–Bonferroni's test was used to draw pairwise multiple comparisons for continuous variables. On the other hand, the proportion difference test was used for the categorical variables.
Finally, patients were categorized based on whether or not they had met the proposed cut-off criterion (GCS, 8 points; PEFR, 160L/m; MEP, 40cmH2O) and the distribution of the progression variables was compared using the Mann–Whitney U test for continuous variables and Fisher's exact test for nominal variables.
All statistical analyses were conducted using IBM SPSS Statistical package for Windows Version 22.0 (Armonk, NY: IBM Corp). p values<.05 were considered significant values.
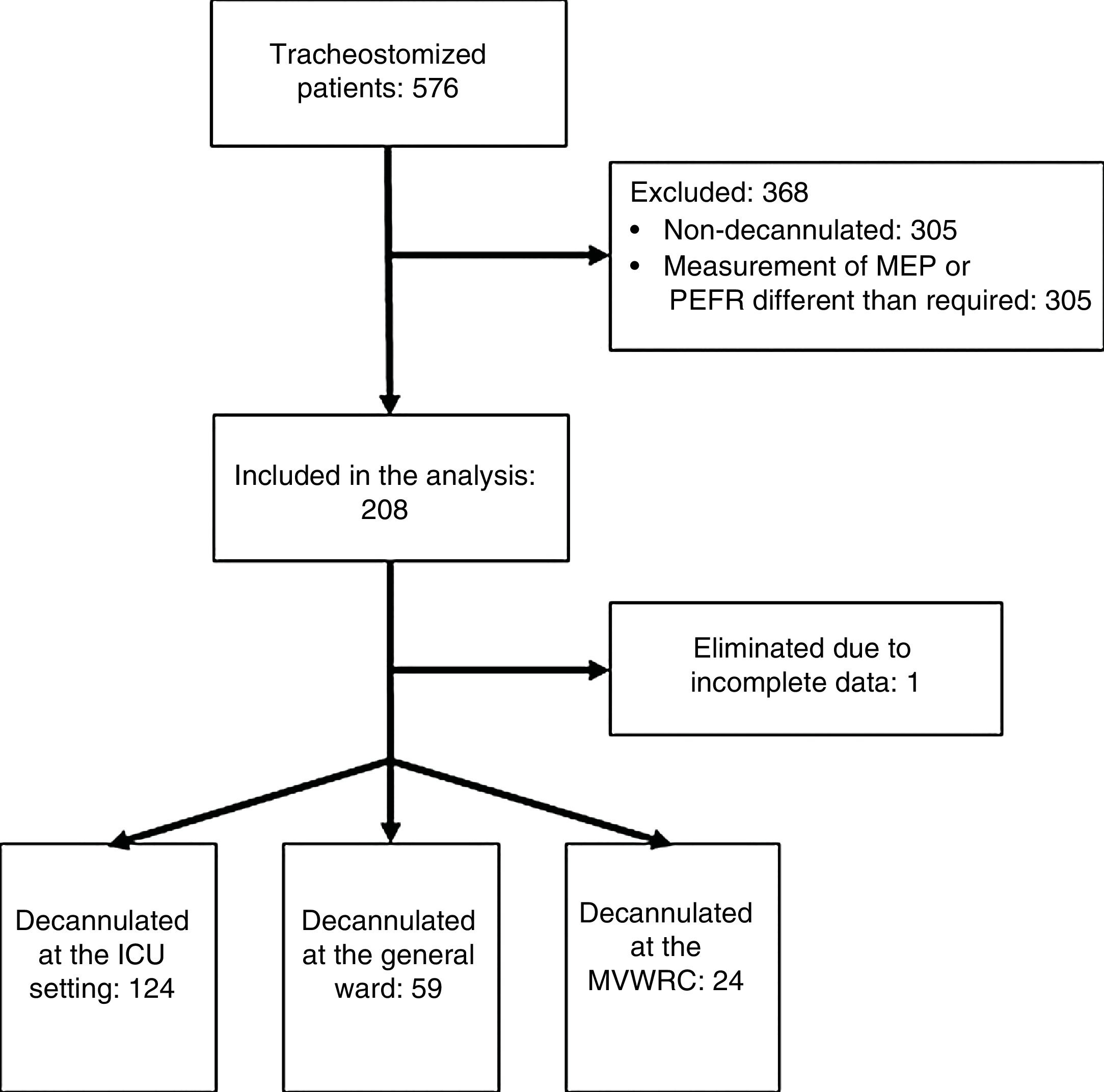
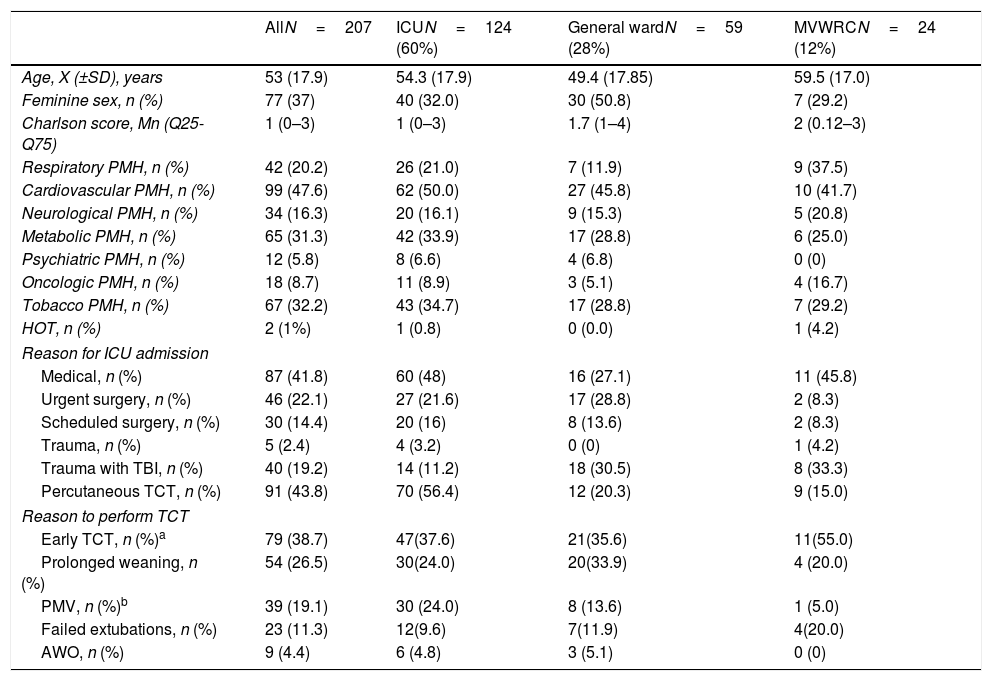
ResultsTwo hundred and seven (207) patients from 36 research centers were studied. Of these, 31 came from ICUs or GWs and 5 from MVWRCs (Annex 1). Fig. 1 shows the flow of patients during the study.
The characteristics of the sample and every independent group are shown on Table 1.
Baseline characteristics of the series.
| AllN=207 | ICUN=124 (60%) | General wardN=59 (28%) | MVWRCN=24 (12%) | |
|---|---|---|---|---|
| Age, X (±SD), years | 53 (17.9) | 54.3 (17.9) | 49.4 (17.85) | 59.5 (17.0) |
| Feminine sex, n (%) | 77 (37) | 40 (32.0) | 30 (50.8) | 7 (29.2) |
| Charlson score, Mn (Q25-Q75) | 1 (0–3) | 1 (0–3) | 1.7 (1–4) | 2 (0.12–3) |
| Respiratory PMH, n (%) | 42 (20.2) | 26 (21.0) | 7 (11.9) | 9 (37.5) |
| Cardiovascular PMH, n (%) | 99 (47.6) | 62 (50.0) | 27 (45.8) | 10 (41.7) |
| Neurological PMH, n (%) | 34 (16.3) | 20 (16.1) | 9 (15.3) | 5 (20.8) |
| Metabolic PMH, n (%) | 65 (31.3) | 42 (33.9) | 17 (28.8) | 6 (25.0) |
| Psychiatric PMH, n (%) | 12 (5.8) | 8 (6.6) | 4 (6.8) | 0 (0) |
| Oncologic PMH, n (%) | 18 (8.7) | 11 (8.9) | 3 (5.1) | 4 (16.7) |
| Tobacco PMH, n (%) | 67 (32.2) | 43 (34.7) | 17 (28.8) | 7 (29.2) |
| HOT, n (%) | 2 (1%) | 1 (0.8) | 0 (0.0) | 1 (4.2) |
| Reason for ICU admission | ||||
| Medical, n (%) | 87 (41.8) | 60 (48) | 16 (27.1) | 11 (45.8) |
| Urgent surgery, n (%) | 46 (22.1) | 27 (21.6) | 17 (28.8) | 2 (8.3) |
| Scheduled surgery, n (%) | 30 (14.4) | 20 (16) | 8 (13.6) | 2 (8.3) |
| Trauma, n (%) | 5 (2.4) | 4 (3.2) | 0 (0) | 1 (4.2) |
| Trauma with TBI, n (%) | 40 (19.2) | 14 (11.2) | 18 (30.5) | 8 (33.3) |
| Percutaneous TCT, n (%) | 91 (43.8) | 70 (56.4) | 12 (20.3) | 9 (15.0) |
| Reason to perform TCT | ||||
| Early TCT, n (%)a | 79 (38.7) | 47(37.6) | 21(35.6) | 11(55.0) |
| Prolonged weaning, n (%) | 54 (26.5) | 30(24.0) | 20(33.9) | 4 (20.0) |
| PMV, n (%)b | 39 (19.1) | 30 (24.0) | 8 (13.6) | 1 (5.0) |
| Failed extubations, n (%) | 23 (11.3) | 12(9.6) | 7(11.9) | 4(20.0) |
| AWO, n (%) | 9 (4.4) | 6 (4.8) | 3 (5.1) | 0 (0) |
AWO, airway obstruction; HOT, home oxygen therapy; ICU, intensive care unit; MVWRC, mechanical ventilation weaning and rehabilitation centers; PMH, past medical history; PMV, prolonged mechanical ventilation; TBI, traumatic brain injury; TCT: tracheostomy.
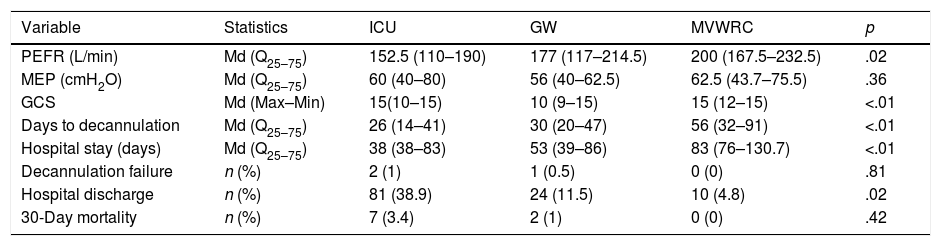
Statistically significant differences were seen in the PEFR and the GCS among the different healthcare settings (p=.028 and p<.01, respectively) (Table 2).
Outcome variables.
| Variable | Statistics | ICU | GW | MVWRC | p |
|---|---|---|---|---|---|
| PEFR (L/min) | Md (Q25–75) | 152.5 (110–190) | 177 (117–214.5) | 200 (167.5–232.5) | .02 |
| MEP (cmH2O) | Md (Q25–75) | 60 (40–80) | 56 (40–62.5) | 62.5 (43.7–75.5) | .36 |
| GCS | Md (Max–Min) | 15(10–15) | 10 (9–15) | 15 (12–15) | <.01 |
| Days to decannulation | Md (Q25–75) | 26 (14–41) | 30 (20–47) | 56 (32–91) | <.01 |
| Hospital stay (days) | Md (Q25–75) | 38 (38–83) | 53 (39–86) | 83 (76–130.7) | <.01 |
| Decannulation failure | n (%) | 2 (1) | 1 (0.5) | 0 (0) | .81 |
| Hospital discharge | n (%) | 81 (38.9) | 24 (11.5) | 10 (4.8) | .02 |
| 30-Day mortality | n (%) | 7 (3.4) | 2 (1) | 0 (0) | .42 |
GCS, Glasgow Coma Score; MEP, maximum espiratory pressure; PEFR, peak expiratory flow rate.
In the progression variables, significant differences were seen in the days wearing the tracheostomy cannula.
In the progression variables, significant differences were found in the days wearing the tracheostomy cannula (ICU, Mn 26 days; GW, Mn 30 days; MVWRC, Mn 56 days, p<.01), the hospital stay (days) (ICU, Mn 53 days; GW, Mn 57 days; MVWRC, Mn 129.5 days, p<.01) and the number of patients who were discharged to their homes (ICU, 81 patients [38.9%]; GW, 24 patients [11.5%]; MVWRC, 10 patients4,8; p=.02). No differences were seen in the number of patients in whom decannulation failed or in the 30-day mortality either.
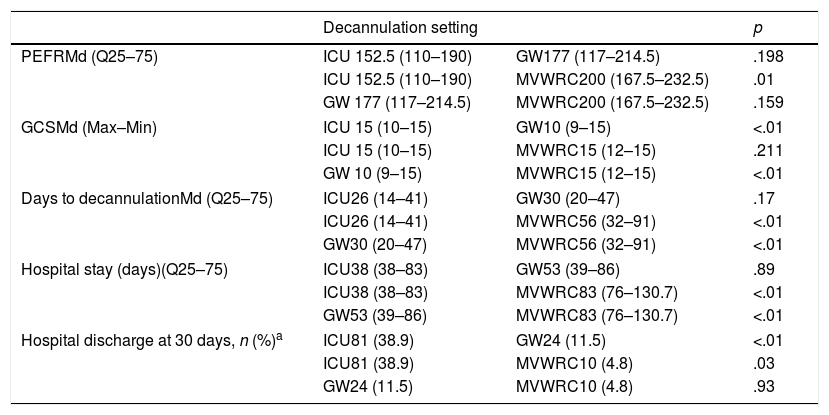
Table 3 shows the post hoc comparisons among the different healthcare settings.
Post hoc analysis.
| Decannulation setting | p | ||
|---|---|---|---|
| PEFRMd (Q25–75) | ICU 152.5 (110–190) | GW177 (117–214.5) | .198 |
| ICU 152.5 (110–190) | MVWRC200 (167.5–232.5) | .01 | |
| GW 177 (117–214.5) | MVWRC200 (167.5–232.5) | .159 | |
| GCSMd (Max–Min) | ICU 15 (10–15) | GW10 (9–15) | <.01 |
| ICU 15 (10–15) | MVWRC15 (12–15) | .211 | |
| GW 10 (9–15) | MVWRC15 (12–15) | <.01 | |
| Days to decannulationMd (Q25–75) | ICU26 (14–41) | GW30 (20–47) | .17 |
| ICU26 (14–41) | MVWRC56 (32–91) | <.01 | |
| GW30 (20–47) | MVWRC56 (32–91) | <.01 | |
| Hospital stay (days)(Q25–75) | ICU38 (38–83) | GW53 (39–86) | .89 |
| ICU38 (38–83) | MVWRC83 (76–130.7) | <.01 | |
| GW53 (39–86) | MVWRC83 (76–130.7) | <.01 | |
| Hospital discharge at 30 days, n (%)a | ICU81 (38.9) | GW24 (11.5) | <.01 |
| ICU81 (38.9) | MVWRC10 (4.8) | .03 | |
| GW24 (11.5) | MVWRC10 (4.8) | .93 | |
All pairs were compared using the Dunn–Bonferroni's test p<.05 except indicated otherwise.
GCS, Glasgow Coma Score; GW, general ward; ICU, intensive care unit; MVWRC, mechanical ventilation weaning and rehabilitation centers; PEFR, peak expiratory flow rate.
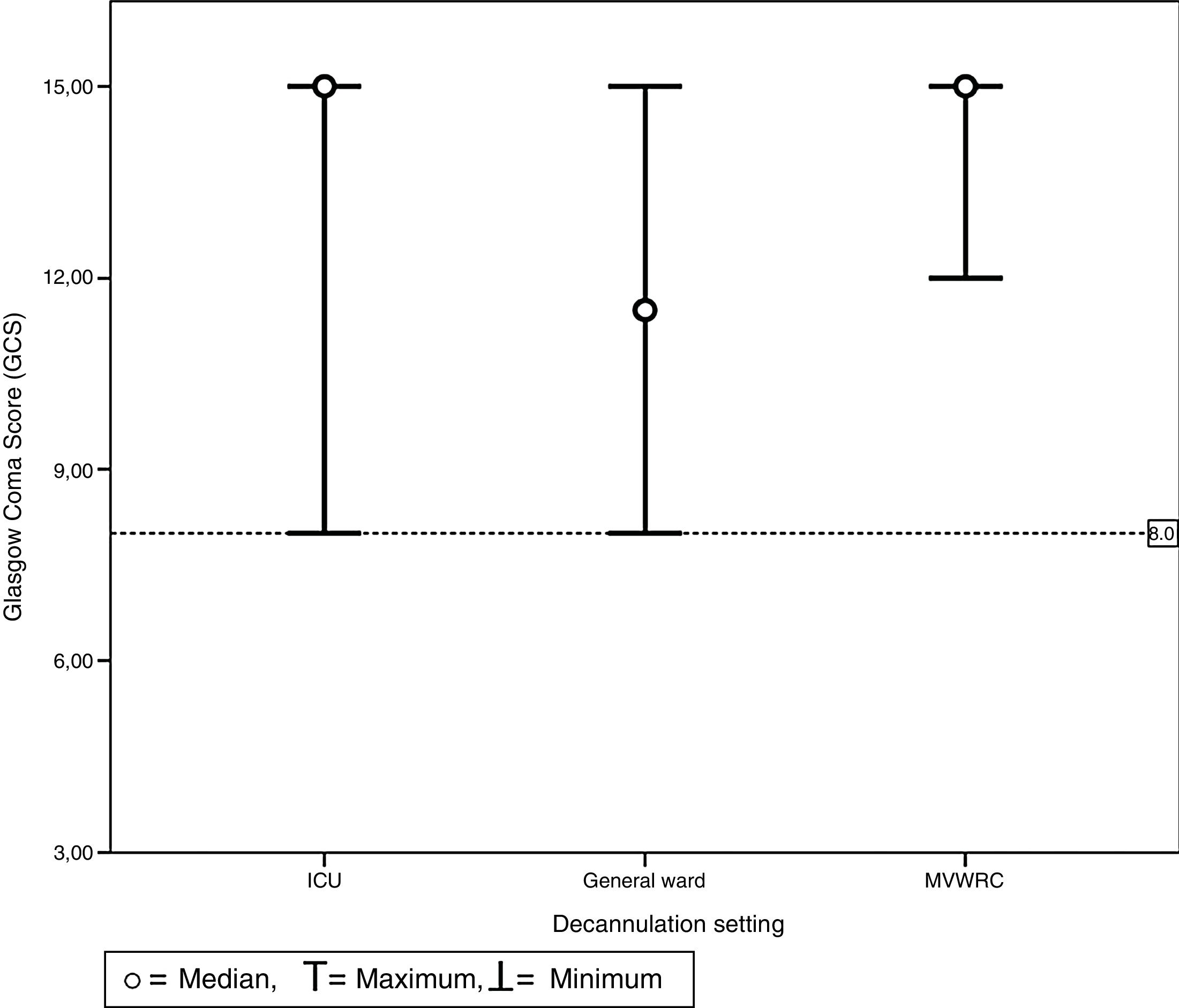
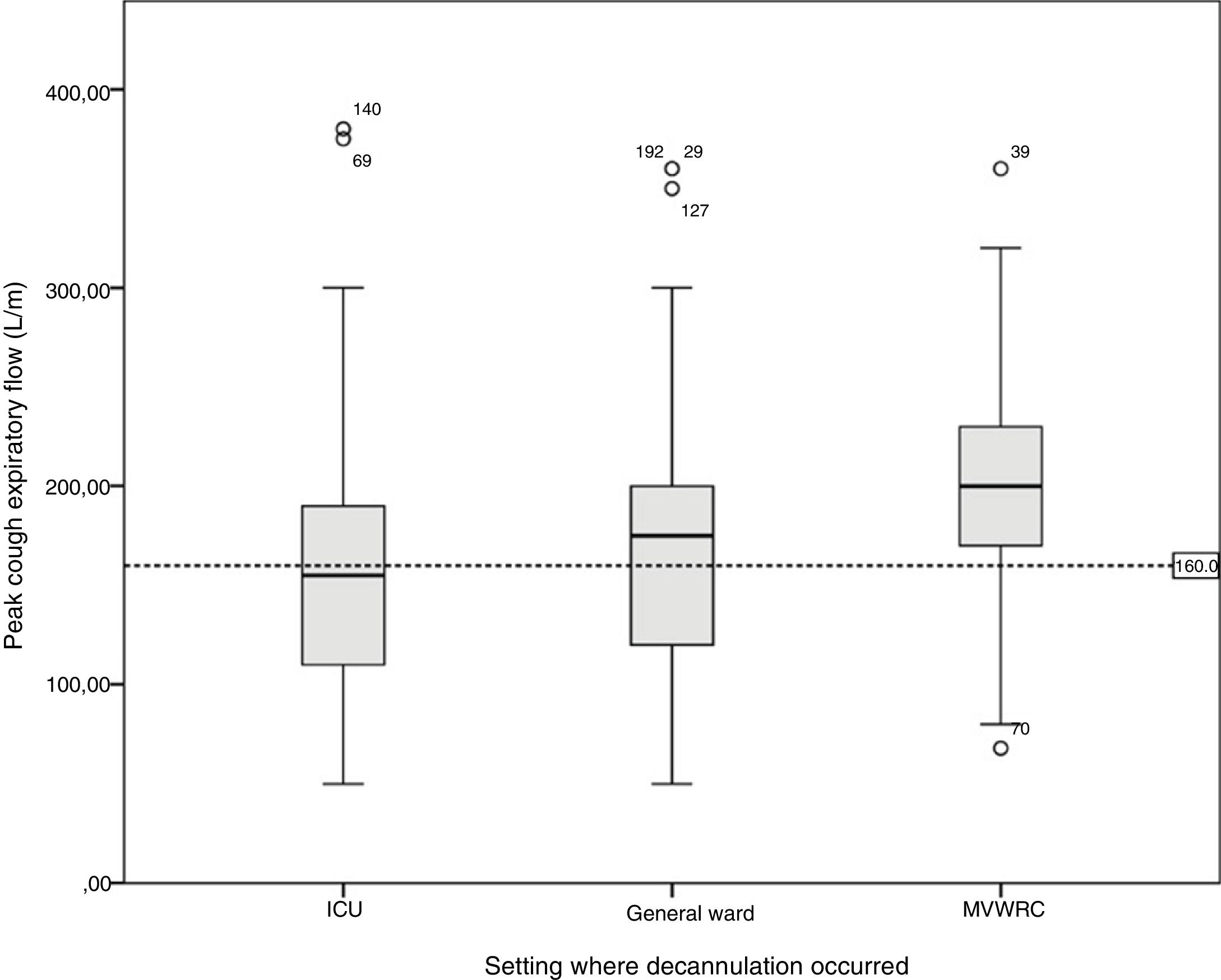
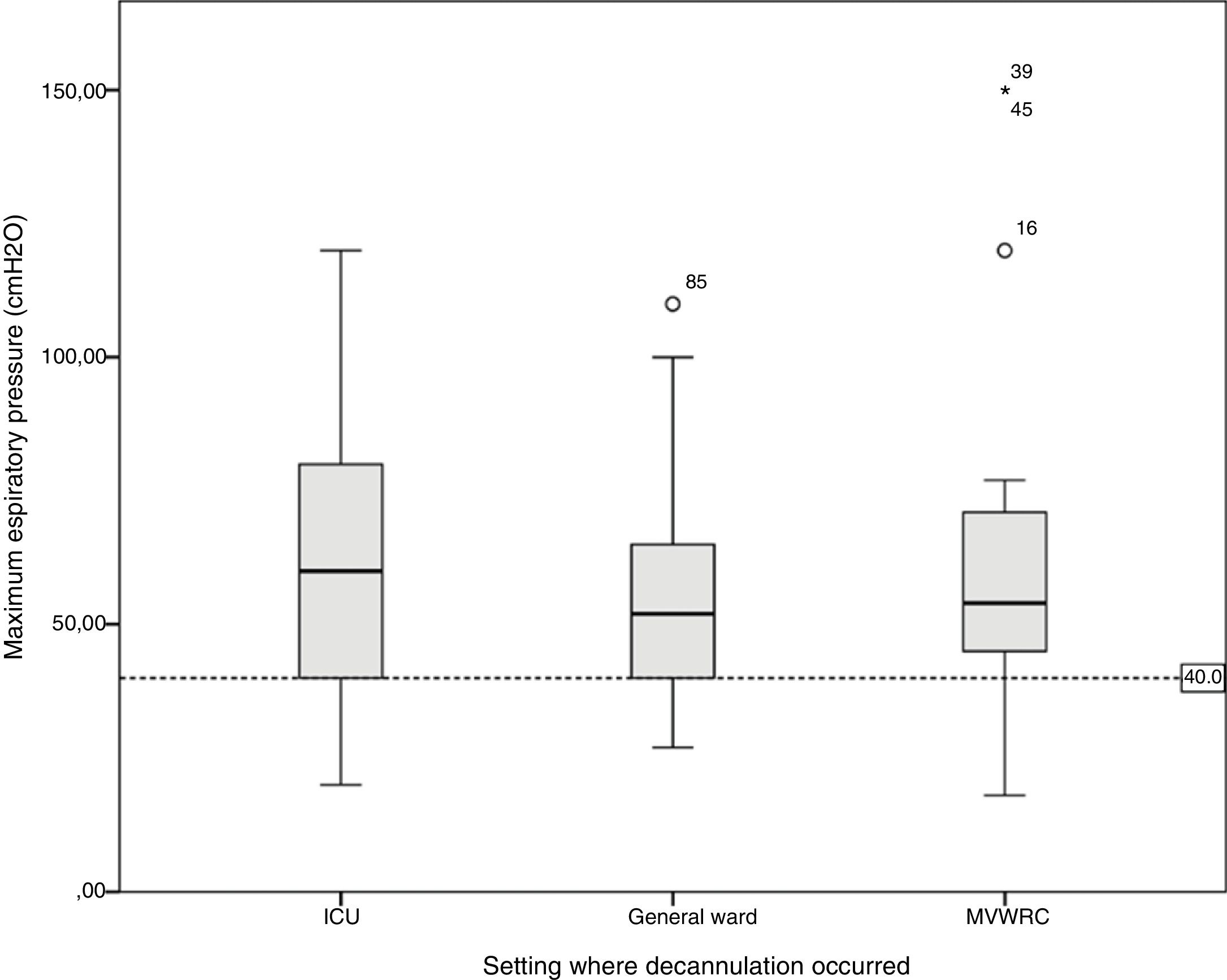
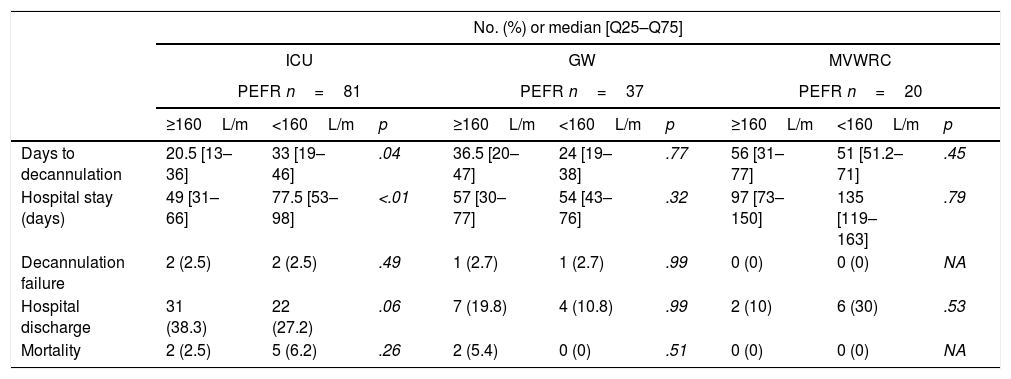
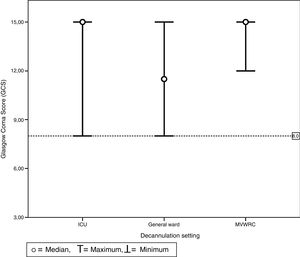
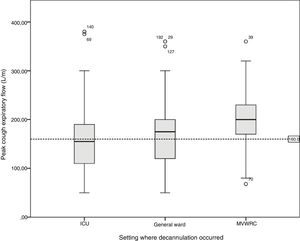
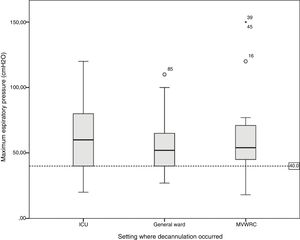
Table 4 shows the comparison of the progression variables by categorizing patients into different groups based on whether or not they had met the cut-off criteria for PEFR and MEP. The GCS variable was not analyzed since no patient was decannulated below the cut-off point established. Also, Fig. 2 shows a graphic representation of the GCS mean with its corresponding minimum and maximum based on the hospitalization setting. Figs. 3 and 4 show the box plot of PEFR and MEP based on the hospitalization setting.
Progression of decannulated patients.
| No. (%) or median [Q25–Q75] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ICU | GW | MVWRC | |||||||
| PEFR n=81 | PEFR n=37 | PEFR n=20 | |||||||
| ≥160L/m | <160L/m | p | ≥160L/m | <160L/m | p | ≥160L/m | <160L/m | p | |
| Days to decannulation | 20.5 [13–36] | 33 [19–46] | .04 | 36.5 [20–47] | 24 [19–38] | .77 | 56 [31–77] | 51 [51.2–71] | .45 |
| Hospital stay (days) | 49 [31–66] | 77.5 [53–98] | <.01 | 57 [30–77] | 54 [43–76] | .32 | 97 [73–150] | 135 [119–163] | .79 |
| Decannulation failure | 2 (2.5) | 2 (2.5) | .49 | 1 (2.7) | 1 (2.7) | .99 | 0 (0) | 0 (0) | NA |
| Hospital discharge | 31 (38.3) | 22 (27.2) | .06 | 7 (19.8) | 4 (10.8) | .99 | 2 (10) | 6 (30) | .53 |
| Mortality | 2 (2.5) | 5 (6.2) | .26 | 2 (5.4) | 0 (0) | .51 | 0 (0) | 0 (0) | NA |
| MEPn=94 | MEPn=48 | MEPn=22 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥40cmH2O | <40cmH2O | p | ≥40cmH2O | <40cmH2O | p | ≥40cmH2O | <40cmH2O | p | |
| Days to decannulation | 25 [13.5–44] | 31 [21.5–49.5] | .17 | 28 [21.2–49] | 32 [18.5–37] | .57 | 51 [31–72] | 140 [132–197] | <.01 |
| Hospital stay (days) | 52 [36.5–90] | 74 [54.5–92] | .17 | 55.5 [39–86.7] | 51 [33–59] | .33 | 123 [67–150] | 221 [200–257] | <.01 |
| Decannulation failure | 4 (4.3) | 0 (0) | .99 | 2 (4.2) | 0 (0) | .99 | 0 (0) | 0 (0) | NA |
| Hospital discharge | 57 (60) | 4 (4.3) | .02 | 18 (37.5) | 2 (4.2) | .56 | 10 (45) | 0 (0) | .22 |
| Mortality | 4 (4.3) | 3 (3.2) | .10 | 2 (4.2) | 0 (0) | .99 | 0 (0) | 0 (0) | NA |
Comparison according to the setting of hospitalization of patients’ progression based on whether or not they met the criterion for decannulation.
GW, general ward; ICU, intensive care unit; MEP, maximum espiratory pressure; MVWRC, mechanical ventilation weaning and rehabilitation centers; PEFR, peak expiratory flow rate.
Chart of maximum and minimum Glasgow Coma Scores prior to decannulation based on the setting where decannulation occurred. No patient was decannulated with GCS below 8 points. This value is represented here by the line which is dotted. This was the same for all settings including the intensive care unit, the general ward, and the mechanical ventilation weaning and rehabilitation center.
Box plot representing the values of the peak cough expiratory flow based on the decannulation setting. The line shows the cut-off value of 160L/min. We can see how this value is reached in different percentages in the different settings: by 46.1% of the patients hospitalized in the intensive care unit, 31.1% of the patients hospitalized in the general ward, and by 17.4% of the patients hospitalized in the mechanical ventilation weaning and rehabilitation center (MVWRC).
Box plot representing the values of maximum expiratory pressure based on the decannulation setting. The line shows the cut-off value of 40cmH2O. We can see how this value is reached in different percentages in the different settings: by 9.7% of the patients hospitalized in the intensive care unit, 5.6% of the patients hospitalized in the general ward, and by 13.0% of the patients hospitalized in the mechanical ventilation weaning and rehabilitation center (MVWRC).
The main finding was that the values of PEFR and MEP for several patients did not meet the cut-off point suggested by the medical literature (160L/min and 40cmH2O, respectively). On the other hand, the cut-off point for the GCS reported by the medical literature was actually respected in all decannulations.1,13,19–22 Also, it was significant to find that the GCS and PEFR values assessed prior to the decannulation procedure were significantly different based on the hospitalization setting where the decannulation occurred.
Contrary to what was observed with the PEFR, the MEP showed differences based on the decannulation setting, which may have to do with the procedure used to obtain both variables. While the measurement of the PEFR has to do with effort, for the measurement of the MEP, one unidirectional valve is used while the patient's voluntary effort is not needed.19–22
The hospitalization setting that showed the lowest PEFR values of all was the ICU. This may be due to the fact that, at the ICU setting, patients are in the process of recovering from acute events and they usually show a highly prevalent condition such as ICU-acquired frailty with accompanied disorders such as peripheral muscle strength and respiratory strength.23–25
For evaluation purposes, all study researchers reported, one PEFR cut-off point of 160L/min as suggested by Bach and Saporito.13 Nevertheless, we saw that in all hospitalization settings, patients with values below the cut-off point were decannulated. Similarly, in a population of neurosurgical postoperative patients, Chan et al. defined PEFR values significantly lower than 29L/min that still ended up leading to successful decannulation procedures.14 The difference between both studies is in the methodology used for the measurements. In Chan et al.’s study the possible air leakage around the cannula was not monitored which may have underestimated the actual PEFR value in these patients. Due to this procedural weakness, we chose as the reference value, the 160L/m value suggested by Bach that, by the way, is widely accepted in the routine daily practice.8,13 Given all this and bearing in mind that the normal rate of decannulation failure is between 0% and 6%,5,8,9,12,26 we believe that the threshold PEFR value to consider it a predictor of successful decannulation should, therefore, be revisited.
Other authors assessed the strength of coughing through the MEP exerted by respiratory muscles. Ceriana et al. prospectively assessed a MEP cut-off point>to 40cmH2O in a decannulation protocol.1 Santus et al. suggest one qualiquantitative algorithm to weigh the capacity to cough assessed through the MEP and use the same threshold to define the pattern.27 In our study there were no differences in the MEP value with respect to decannulation rate among the different healthcare settings studied. This contradictory finding may be explained by what we’ve just explained above when it comes to the use of the unidirectional valve method to measure the MEP.
The differences observed in the GCS between the GW compared to the ICU and the MVWRC suggest that in the GW setting lower GCS values are tolerated for decannulation purposes compared to the ICU. We believe that this observation may show different stages in the progression of a patient who has left his critical stage behind. One may argue that patients who leave the ICU for an inpatient hospital room already had failed decannulation attempts. This may condition a state of consciousness with lower scores when it is time to decannulate. Also, this may vary in tracheostomized patients who are referred to a MVWRC where the therapeutic goals and the hospital stays are different compared to the GW setting. Also, the patient is re-categorized as a chronically ill patient, which may result in more strict criteria for decannulation. This issue has not been discussed in any the references from the medical literature yet.
In our study, the GCS at the moment of decannulation was not below the cut-off point chosen in any of the settings evaluated. This finding reinforces the claims made by several authors such as Stelfox et al. who gathered the opinion of experts on the management of tracheostomized patients being the state of consciousness one of the determinants for safe decannulation procedures.8 Hernandez et al. found longer decannulation times in patients with GCS<13 points28. In another study among neurosurgical patients, Chan et al. assessed the GCS prior to decannulation, and they associated it with successful decannulations. The authors conclude that although the GCS does not increase the risk of decannulation failure, there is a certain trend that suggests that patients who failed showed lower GCS (p=.06).14 In sum, even though the observed or chosen values to define the state of consciousness vary from one author to the other, all of them take the GCS-defined state of consciousness into account when performing safe decannulation procedures.
Progression variables in the series of patients decannulated showed the anticipated results. Mortality and decannulation failure were not significant among the different settings. This is consistent with what other authors claim about patients who have been successfully weaned and who are eligible for decannulation, that the result of the decannulation procedure is not influenced by the treating center.27 Contrary to this claim there are studies that attribute higher mortality rates to decannulations performed in the general ward.28,29
Both the hospital stays and the days to decannulation were significantly and numerically higher in the general ward compared to the MVWRC. This may be explained by the fact that these are usually referral settings that typically follow the ICU stay.
Finally, when comparing the patients’ progression based on whether they had been decannulated following the criteria proposed by the medical literature,1,8,13,27 we observed that those who did not meet these parameters had longer hospital stays, more decannulation days, and that a greater number of these patients were discharged from the hospital. However, this difference was not statistically significant among the different hospital settings.
This study has some limitations that should be taken into consideration here. In the first place, there is no control group (decannulated patients) to make comparisons among the variables commonly used to guide decannulation and then be able to define the diagnostic criteria of the variables studied. Another limitation would be the variability of the devices used to measure the peak cough expiratory flow, since several centers lacked pneumotachographs to measure the peak flow and used mechanical sensors instead. On the other hand, the PEFR values may have been underestimated since peristomal leakage at the moment of the measurement was not monitored, unlike what happened with the MEP where the balloon was overinflated to take the measurements.
Yet despite the limitations exposed here we believe that this study is an important one because of the data presented that are used on a daily basis by high-ranking centers for the management of tracheostomized patients. Although decannulation may by influenced by a great number of variables, several authors agree that the strength of coughing and the state of consciousness are usually the most important variables when it comes to making decannulation decisions.1,8,27
These findings should be assessed prospectively through controlled trials in order to establish efficacy criteria for the variables suggested. We believe that it is important to assess the safety of decannulation while taking into account that unnecessary long tracheostomy cannulation times may bring complications.
ConclusionIn this study we saw differences in the PEFR and GCS values among the different hospital settings that perform traqueostomy cannula removals. A significant number of patients were decannulated with PEFR and MEP values below the cut-off points suggested by the medical literature as predictors of decannulation failure. No patient in our series was decannulated with GCS below 8 points, indicative of how important the state of consciousness really is when making the decision to decannulate. The follow-up of this series shows the prolonged time of the presence of a tracheostomy cannula and that decannulation is associated with greater chances of being discharged from the hospital.
Authors/collaboratorsLadislao Díaz Ballve participated in the study idea and design, data mining, and in the analysis and interpretation of data. Also, he conducted the paper draft or the critical review of the intellectual content and participated in the definitive approval of the final version.
Darío Villalba participated in the study idea and design, data mining, and in the analysis and interpretation of data. Also, he conducted the paper draft or a critical review of the intellectual content and participated in the definitive approval of the final version.
Mauro Andreu participated in the study idea and design, data mining, and in the analysis and interpretation of data. Also, he conducted the paper draft or the critical review of the intellectual content and participated in the definitive approval of the final version.
Miguel Escobar participated in the study idea and design, data mining, and in the analysis and interpretation of data. Also, he conducted the critical review of the intellectual content and participated in the definitive approval of the final version.
Gastón Morel Vulliez participated in the study idea and design, data mining, and in the analysis and interpretation of data. Also, he conducted the critical review of the intellectual content and participated in the definitive approval of the final version.
Janina Lebus participated in the study idea and design, data mining, and in the analysis and interpretation of data. Also, he conducted the critical review of the intellectual content and participated in the definitive approval of the final version.
Emilio Rositi participated in the study idea and design, data mining, and in the analysis and interpretation of data. Also, he conducted the critical review of the intellectual content and participated in the definitive approval of the final version.
Conflicts of interestThe authors declare no conflicts of interest whatsoever.
Researchers and their respective centers
Díaz-Ballve Ladislao and Turón Gonzalo (Hospital Nacional Alejandro Posadas, El Palomar, Buenos Aires); Villalba Darío (Clínica Basilea, CABA); Escobar Miguel, Morel-Vulliez Gastón, Rositi Emilio and Gussoni Mariana (Centro Parque, CABA); Lebus Janina (Clínica La Sagrada Familia, CABA); Falduti Alejandra (Hospital Juan A. Fernández, CABA); Tenasczuk Karina (Alta Complejidad en Red, Hospital El Cruce, Dr. Néstor C. Kirchner, Lomas de Zamora, Buenos Aires); Reinoso Mayra (Hospital Italiano de Buenos Aires, CABA); Quijano Agustina and Bustamante Paola (Clínica de Internación Aguda en Rehabilitación y Cirugía [CIAREC], CABA); Di Pierro Mercedes (Hospital Regional Dr. Ramón Carrillo, Ciudad de Santiago del Estero, Santiago del Estero); Santini Marcela (Clínica Pasteur, Ciudad de Neuquén, Neuquén); Borello Silvina and Aguirre Mariana (Hospital Donación Francisco Santojanni, CABA), Gracia Guadalupe (Sanatorio Colegiales, CABA); Setten Mariano (Centro de Educación Médica e Investigaciones Clínicas «Norberto Quirno» [CEMIC], CABA); Di-Nardo Soledad (Clínica Santa Isabel, CABA); Navarro Emiliano (Hospital Carlos G. Durand, CABA); Ruggeri Federico (Hospital Escuela José de San Martín, Cdad. de Corrientes Provincia de Corrientes); Camargo Marcel, Franco Leandro, Funes Juan, García Luciano and Sosa Adriana (Hospital San Luis, Ciudad de San Luis, San Luis); Uberti Mariano (Clínica San Agustín, Ciudad de Neuquén, Neuquén); Mogadouro Mariela (Sanatorio de La Trinidad Palermo, CABA); Rapetti Leticia (Hospital Universitario UAI, CABA); Baqueiro Ayelen (Hospital Regional Artémides Zatti, Viedma, Río Negro); Garzón Gustavo (Sanatorio Nuestra Señora del Rosario, San salvador de Jujuy, Jujuy); Cervantes Violeta (Hospital María Ferrer, CABA); Revelli Rosana (Hospital Privado Universitario, Cdad. de Córdoba, Córdoba); Moreno Martín (Hospital Regional, Comodoro Rivadavia, Chubut); Hassan Ana Paula (Hospital Zonal, Esquel, Chubut); Busico Marina (Clínica Olivos - SMG, Olivos, Provincia de Buenos Aires); Luponio Marcelo (Hospital Julio C. Perrando, Resistencia, Chaco); Gelabert Deborah (Hospital Óscar Alende, Mar del Plata, Buenos Aires); González Luis (Clínica ALCLA, CABA); Rojas Vanesa (HIGA Petrona Villegas de Cordero, San Fernando, Buenos Aires); Kaspar Guillermina (Instituto de Investigaciones Médicas Alfredo A. Lanari, CABA); Veronesi Magdalena (Clínica Altergarten, CABA); Verduguez Marta (Hospital Simplemente Evita, González Catán, Buenos Aires); Seguil Yanina (Hospital Municipal de Trauma y Emergencias Dr. Federico Abete. Malvinas Argentinas, Buenos Aires); Terán Eduardo (Hospital Central, Ciudad de Mendoza, Mendoza).
Please cite this article as: Diaz-Ballve LP, Villalba DS, Andreu MF, Escobar MA, Morel-Vulliez G, Lebus JM, et al. Valores de fuerza muscular respiratoria y estado de conciencia medido previo a la decanulación en diferentes niveles de complejidad. Estudio de serie de casos longitudinal y prospectiva. Med Intensiva. 2019;43:270–280.