To analyze the hematological complications and need for transfusions in children receiving extracorporeal life support (ECLS).
DesignA retrospective study was carried out.
SettingA pediatric intensive care unit.
PatientsChildren under 18 years of age subjected to ECLS between September 2006 and November 2015.
InterventionsNone.
Variables of interestPatient and ECLS characteristics, anticoagulation, hematological and coagulation parameters, transfusions and clinical course.
ResultsA total of 100 patients (94 with heart disease) with a median age of 11 months were studied. Seventy-six patients presented bleeding. The most frequent bleeding point was the mediastinum and 39 patients required revision surgery. In the first 3days, 97% of the patients required blood transfusion (34.4ml/kg per day), 94% platelets (21.1ml/kg per day) and 90% plasma (26.6ml/kg per day). Patients who were in the postoperative period, those who were bleeding at the start of ECLS, those requiring revision surgery, those who could not suspend extracorporeal circulation, and those subjected to transthoracic cannulation required a greater volume of transfusions than the rest of the patients. Thromboembolism occurred in 14 patients and hemolysis in 33 patients. Mortality among the children who were bleeding at the start of ECLS (57.6%) was significantly higher than in the rest of the patients (37.5%) (P=0.048).
ConclusionsChildren subjected to ECLS present high blood product needs. The main factors related to transfusions were the postoperative period, bleeding at the start of ECLS, revision surgery, transthoracic cannulation, and the impossibility of suspending extracorporeal circulation. Children with bleeding suffered greater mortality than the rest of the patients.
Analizar las complicaciones hematológicas y las necesidades transfusionales en niños tratados con oxigenación por membrana extracorpórea (ECMO).
DiseñoEstudio retrospectivo.
ÁmbitoUnidad de cuidados intensivos pediátricos.
PacientesNiños menores de 18 años tratados con ECMO entre septiembre de 2006 y noviembre de 2015.
IntervencionesNinguna.
Variables de interésCaracterísticas clínicas, de la ECMO, anticoagulación, parámetros hematológicos y de coagulación, transfusiones y evolución clínica.
ResultadosSe estudiaron 100 pacientes con una mediana de edad de 11 meses. Presentaron sangrado 76; el mediastino fue la localización más frecuente; 39 precisaron revisión quirúrgica. En los primeros 3 días de ECMO, el 97% de los pacientes precisaron transfusión de hematíes (34,4 ml/kg al día), el 94% plaquetas (21,1ml/kg al día) y el 90% plasma (26,6ml/kg al día). Los pacientes posquirúrgicos, con imposibilidad de salida de la circulación extracorpórea, los que presentaron sangrado al inicio de la ECMO, los que precisaron revisión quirúrgica y los que tuvieron canulación transtorácica requirieron mayor volumen de transfusiones. Se produjeron tromboembolias en 14 pacientes y hemólisis en 33. La mortalidad de los niños que presentaron sangrado al inicio de ECMO (57,6%) fue significativamente mayor que la del resto (37,5%) (p = 0,048).
ConclusionesLos niños tratados con ECMO presentan una elevada incidencia de sangrado y precisan un gran volumen de transfusiones. El postoperatorio de cirugía, el sangrado al inicio de la ECMO, la necesidad de revisión quirúrgica, la imposibilidad de salida de la circulación extracorpórea y la canulación transtorácica se asocian a un mayor volumen de transfusiones. Los niños que sangraron al inicio de la ECMO presentaron mayor mortalidad.
Extracorporeal membrane oxygenation (ECMO) is a circulatory support technique associated with a high risk of complications – the most frequent being of a hemodynamic, neurological and hematological nature.1–4
The most important hematological complications consist of bleeding and thromboembolism,4–7 which are responsible for up to 30–40% of all deaths among patients subjected to ECMO.8,9
Bleeding is the most frequent and serious complication in children subjected to ECMO, particularly in neonates and in surgical patients.10 Anticoagulation with heparin, needed to prevent thrombus formation, increases the risk of bleeding.11 It is also common to detect thrombocytopenia resulting from the activation and depletion of platelets due to multiple causes.1 For these reasons, children subjected to ECMO receive numerous blood transfusions.6
The primary objective of this study was to analyze the incidence and factors predisposing to hematological complications and the transfusion requirements in children subjected to ECMO. The secondary objective was to examine the relationship between these complications and mortality and the duration of admission to the Pediatric Intensive Care Unit (PICU).
Patients and methodsA retrospective study was made of a prospective database including all children under 18 years of age subjected to ECMO between September 2006 and November 2015. There were no exclusion criteria. The study was approved by the Local Ethics Committee. In all cases ECMO involved the use of a centrifugal pump (Rotaflow, Maquet®) and a hollow polymethylpentene fiber oxygenator (Quadrox, Maquet®). In the case of patients weighing under 15kg, we used a Quadrox-iD Pediatr® oxygenator with a membrane surface of 0.8m2 and a priming volume of 81ml allowing a blood flow of between 0.2 and 2.8l/min. In the case of patients weighing over 15kg we used the Quadrox-D® model with a membrane surface of 1.8m2 and a priming volume of 250ml allowing a blood flow of between 0.5 and 7l/min. There were two types of pediatric circuits: 1/4in. (neonates and nursing infants) and 3/8in. Fig. 1 of the supplementary material schematically shows the circuit used. Cervical cannulation was the option of choice. Transthoracic cannulation was decided in cases where extracorporeal life support could not be suspended after surgery, in patients with recent sternotomy, and in situations of extreme emergency.
Anticoagulation was provided with sodium heparin in continuous infusion at a dose of 10–50IU/kgh−1. The control of anticoagulation and modification of the heparin dose throughout the study was based on the combined use of activated coagulation time (ACT) at the patient bedside every 30–60min, activated partial thromboplastin time (aPTT) (measured at least once a day) and the levels of antifactor Xa (antiXa), which were used as reference value. We recorded data referred to the patient (age, weight, diagnosis), ECMO (indication, type and duration), anticoagulation (heparin dose), the hematological and coagulation parameters (hemoglobin [Hb], platelets, international normalized ratio [INR], ACT, aPTT, fibrinogen, antiXa, free Hb), the administration of transfusions (packed red cells, plasma and platelets) and the clinical course (bleeding complications, thrombosis, hemolysis, duration of admission to the PICU, survival).
Significant bleeding was defined as any bleeding causing a decrease in hemoglobin of over 2g/dl in 24h, or which required transfusion. Significant hematological alterations requiring blood product transfusions were defined as Hb<9g/dl, platelets<100,000/mm3, INR and aPTT alteration>2 times the control level, and fibrinogen<150mg/dl. These values were regarded as general indicators of transfusion, but the decision was made on an individualized basis in each patient, taking into account the clinical condition, bleeding volume and hematological alterations.
The study was divided into two equal periods in order to analyze the possible existence of differences in the course of the study.
The statistical analysis of the data was carried out using the SPSS version 21.0 statistical package. Quantitative variables with a normal distribution were reported as the mean and standard deviation (SD), while those exhibiting a non-normal distribution were reported as the median and interquartile range (IQR) (p25–p75). Qualitative variables were reported as percentages. The chi-squared test or Fisher exact test was used for the comparison of qualitative variables, while quantitative variables were compared by the Student's t-test and Wilcoxon rank-sign test, according to whether or not the data exhibited a normal distribution. Multivariate logistic regression analysis was performed to assess the association between each of the factors and the need for packed red cell, platelet and plasma transfusions. Statistical significance was considered for p<0.05.
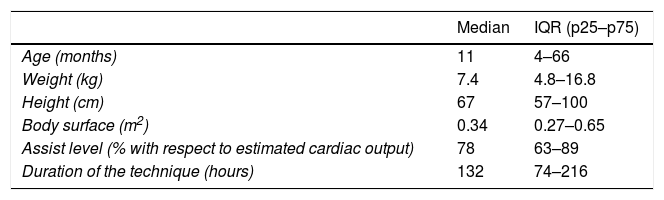
ResultsThe study comprised a total of 100 patients (67% males) with a median age of 11 months (IQR 4–66 months) and a median weight of 7.4kg (IQR 4.8–16.8kg). The diagnosis was heart disease in 94 patients (64 were in the postoperative period of heart surgery), respiratory failure in 5 patients and septic shock in one. The indication of ECMO was the impossibility of suspending extracorporeal life support in 27 patients, postoperative low cardiac output in 23 cases, non-postoperative severe heart failure in 18 patients, cardiac arrest in 12 and refractory arrhythmias in 7 patients.
Venoarterial ECMO was used in 98 patients and venovenous ECMO in two cases. Cervical cannulas were placed in 55 patients and transthoracic cannulas in 30, while both types of cannulation were used in 15 cases. Table 1 summarizes the general patient data.
Characteristics of the patients and ECMO.
| Median | IQR (p25–p75) | |
|---|---|---|
| Age (months) | 11 | 4–66 |
| Weight (kg) | 7.4 | 4.8–16.8 |
| Height (cm) | 67 | 57–100 |
| Body surface (m2) | 0.34 | 0.27–0.65 |
| Assist level (% with respect to estimated cardiac output) | 78 | 63–89 |
| Duration of the technique (hours) | 132 | 74–216 |
| Percentage (%) | |
|---|---|
| Gender (male) | 67 |
| Diagnosis | |
| Heart disease | 94 |
| Respiratory failure | 5 |
| Septic shock | 1 |
| Indication | |
| Non-postoperative low output | 18 |
| Postoperative low output | 23 |
| Hypoxemia | 13 |
| No suspension of ECC | 27 |
| Cardiac arrest | 12 |
| arrhythmia | 7 |
| Immediate postoperative period of heart surgery | 64 |
| Type of ECMO | |
| Venoarterial | 98 |
| Venovenous | 2 |
| Type of cannulation | |
| Cervical | 55 |
| Transthoracic | 30 |
| Both | 15 |
ECC: extracorporeal circulation; IQR: interquartile range.
Hemoglobin decreased progressively up until day 7, and recovered thereafter. Statistically significant differences were only observed among the hemoglobin levels at 24h, 72h and 5 days (Fig. 2 of the supplementary material). The platelet counts decreased up until day 7 and remained stable thereafter. There were significant differences between the baseline platelet count and the counts during the rest of the clinical course (Fig. 3 of the supplementary material).
The INR was initially prolonged but gradually normalized over time (Fig. 4 of the supplementary material), while fibrinogen remained within normal ranges in most of the patients from the start of ECMO (data not shown).
Ten percent of the patients presented hemoglobin<9g/dl; 30% had a platelet count of<100,000/mm3; 19% showed INR>2; and 11% presented fibrinogen<150mg/dl.
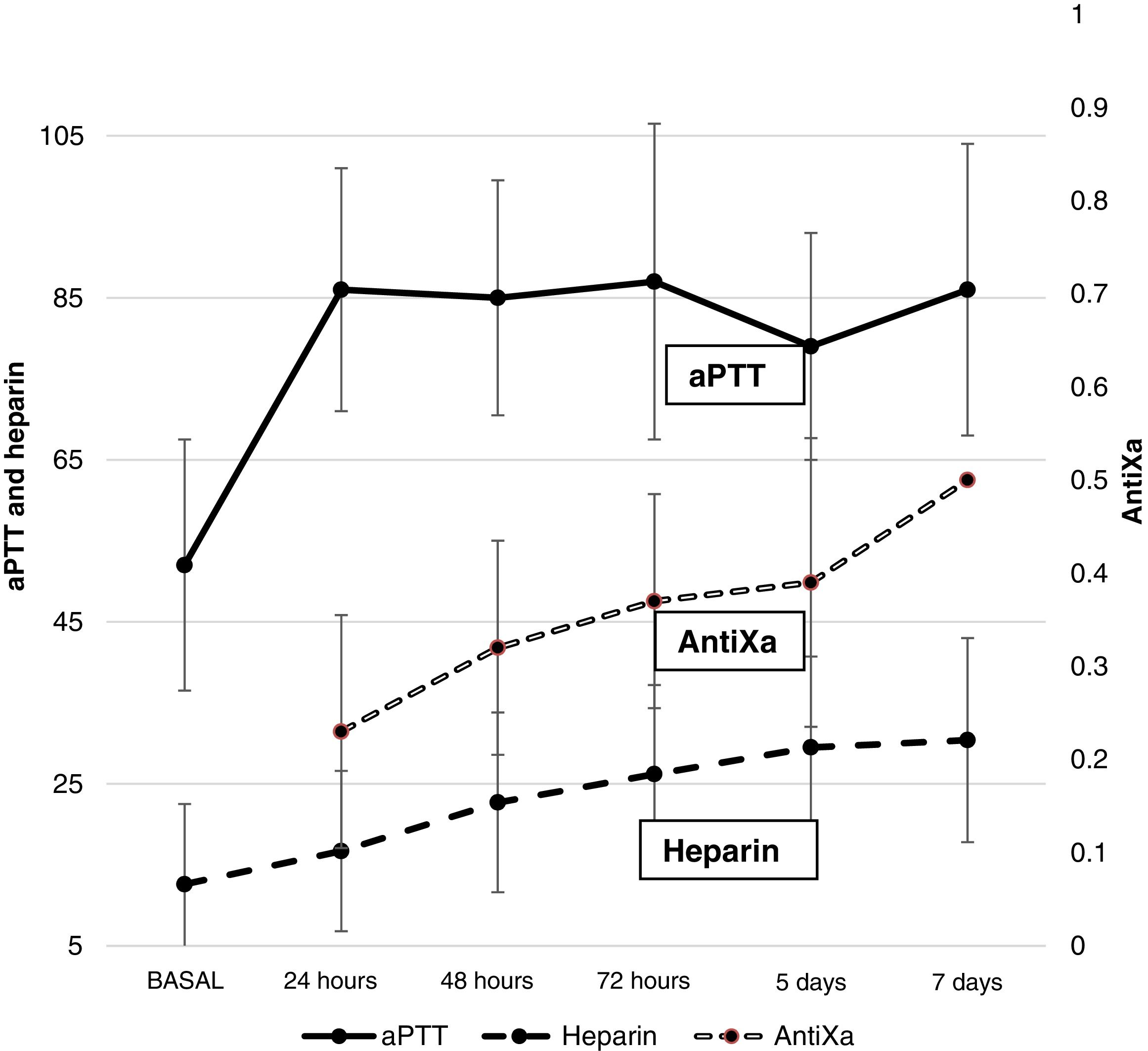
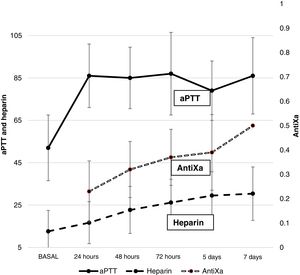
AnticoagulationFig. 1 shows the heparin dose and values corresponding to aPTT and antiXa. A gradual increase in heparin requirements was observed during ECMO. The aPTT values increased early after the start of anticoagulation and remained high over the entire time course. Only 22.2% of the patients had antiXa within therapeutic ranges on the first day – this objective being reached on day 5 of ECMO.
BleedingBleeding was recorded in 52 patients in the first 24h and in 76 patients in the global course of treatment. Another 39 patients required revision surgery due to bleeding. The most frequent location of bleeding was the mediastinum (34 patients), with resulting tamponade in 15 patients. Diffuse bleeding was observed in 14 patients, another 14 suffered lung bleeding, 6 presented peri-cannula bleeding, 5 digestive bleeding, and three suffered cerebral hemorrhage.
The incidence of bleeding in the first 24h among the patients in the postoperative period of surgery (67.2%) was significantly higher than in the rest of the children (25%) (p=0.001).
Those patients with fibrinogen<150mg/dl and a platelet count<100,000/mm3 had a higher incidence of initial bleeding (81.8% and 66.7%) than those with fibrinogen>150 and platelets>100,000/mm3 (48.3% and 45.7%), but the differences failed to reach statistical significance (p=0.053 and p=0.080, respectively). There were no significant differences in the incidence of bleeding at the start of ECMO between the children with INR>2 (52.6%) and those with INR<2 (51.9%) (p=1).
TransfusionsIn the first 24h of ECMO, 85 patients received red cell transfusions, 78 received platelet infusions, and 83 received plasma. The red cell transfusion volume during the first three days of ECMO was 34.4ml/kg/day (SD 31.1), the platelet volume was 21.1ml/kg/day (SD 19.1), and the plasma volume was 26.6ml/kg/day (SD 24.8).
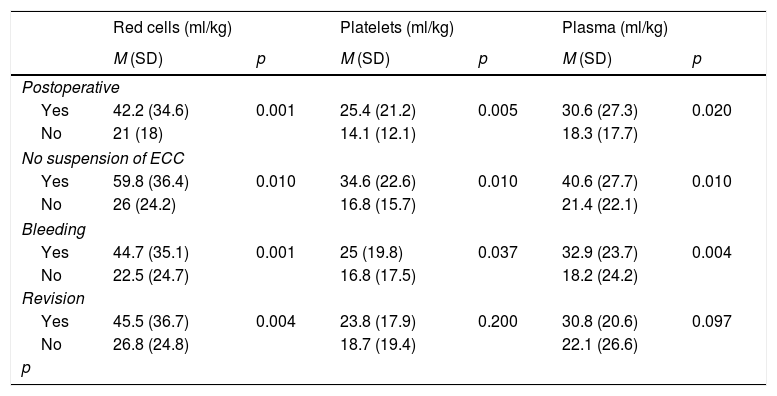
The children in the postoperative period of heart surgery, those in which ECMO was indicated due to the impossibility of suspending extracorporeal life support, those with initial bleeding, and the children requiring revision surgery needed significantly greater transfusion volumes (Table 2).
Factors related to the need for transfusions.
| Red cells (ml/kg) | Platelets (ml/kg) | Plasma (ml/kg) | ||||
|---|---|---|---|---|---|---|
| M (SD) | p | M (SD) | p | M (SD) | p | |
| Postoperative | ||||||
| Yes | 42.2 (34.6) | 0.001 | 25.4 (21.2) | 0.005 | 30.6 (27.3) | 0.020 |
| No | 21 (18) | 14.1 (12.1) | 18.3 (17.7) | |||
| No suspension of ECC | ||||||
| Yes | 59.8 (36.4) | 0.010 | 34.6 (22.6) | 0.010 | 40.6 (27.7) | 0.010 |
| No | 26 (24.2) | 16.8 (15.7) | 21.4 (22.1) | |||
| Bleeding | ||||||
| Yes | 44.7 (35.1) | 0.001 | 25 (19.8) | 0.037 | 32.9 (23.7) | 0.004 |
| No | 22.5 (24.7) | 16.8 (17.5) | 18.2 (24.2) | |||
| Revision | ||||||
| Yes | 45.5 (36.7) | 0.004 | 23.8 (17.9) | 0.200 | 30.8 (20.6) | 0.097 |
| No | 26.8 (24.8) | 18.7 (19.4) | 22.1 (26.6) | |||
| p | ||||||
ECC: extracorporeal circulation; SD: standard deviation; M: mean; Postoperative: postoperative period of heart surgery; Revision: revision surgery; Bleeding: initial bleeding.
The multivariate logistic regression analysis showed the factors significantly associated to a need for packed red cell transfusions to be the impossibility of suspending extracorporeal life support as an indication of ECMO (odds ratio [OR] 23.43; 95% confidence interval [95%CI]: 6.05–40.80; p=0.009) and transthoracic cannulation (OR 21.10; 95%CI: 6.08–36.11; p=0.007). In the multivariate analyses of the need for platelet and plasma transfusions, none of the studied factors reached statistical significance.
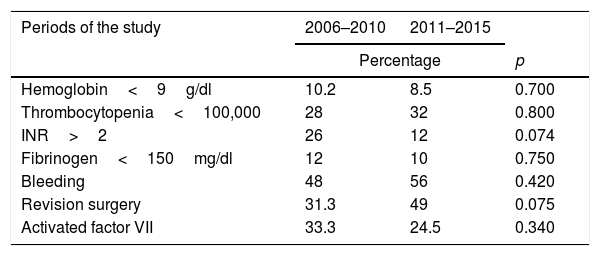
There were no significant differences in the incidence of hematological alterations or in the transfusion needs between the two periods of the study (Table 3).
Comparison of the hematological alterations and transfusion needs in the two periods of the study.
| Periods of the study | 2006–2010 | 2011–2015 | |
|---|---|---|---|
| Percentage | p | ||
| Hemoglobin<9g/dl | 10.2 | 8.5 | 0.700 |
| Thrombocytopenia<100,000 | 28 | 32 | 0.800 |
| INR>2 | 26 | 12 | 0.074 |
| Fibrinogen<150mg/dl | 12 | 10 | 0.750 |
| Bleeding | 48 | 56 | 0.420 |
| Revision surgery | 31.3 | 49 | 0.075 |
| Activated factor VII | 33.3 | 24.5 | 0.340 |
| Mean (SD) | |||
|---|---|---|---|
| Red cell transfusion (ml/kg/day) | 34.4 (36) | 34.2 (25.5) | 0.980 |
| Platelet transfusion (ml/kg/day) | 21.2 (24) | 21.1 (12.5) | 0.980 |
| Plasma transfusion (ml/kg/day) | 26.5 (29.1) | 25.4 (19.8) | 0.890 |
INR: international normalized ratio.
Fourteen thromboembolic events were recorded. The location was peripheral in 6 patients, with acral zone embolism. These 6 patients died under conditions of multiorgan failure. Three patients suffered cerebral thromboembolism, with secondary infarction; one of these patients died.
The ultrasound controls made due to malfunctioning of the ECMO circuit diagnosed a right atrial thrombus, a thrombus in the inferior vena cava, and another in the aorta. One of these patents suffered pulmonary embolism. Peritoneal embolism was suspected in one subject due to suspected intestinal ischemia. Five patients suffered a second thromboembolic event. Twenty-one patients developed thrombi in the oxygenator. In 10 of them thrombosis affected the arterial side of the oxygenator or caused it to malfunction – thereby requiring replacement of the circuit.
HemolysisThe evolution of the free hemoglobin levels is reported in Fig. 5 of the supplementary material. Excessive hemolysis (free Hb>50mg/dl) was observed in 33 patients. The mean time from the start of ECMO to the appearance of hemolysis was 3.9 days (SD 2). Five circuit replacements were required because of hemolysis.
The patients with hemolysis were younger and weighed less than the rest: 20.1 months (SD 35.3) versus 46.9 months (SD 55.4) (p=0.013) and 7.7kg (SD 5.9) versus 16.4kg (SD 18.4) (p=0.01).
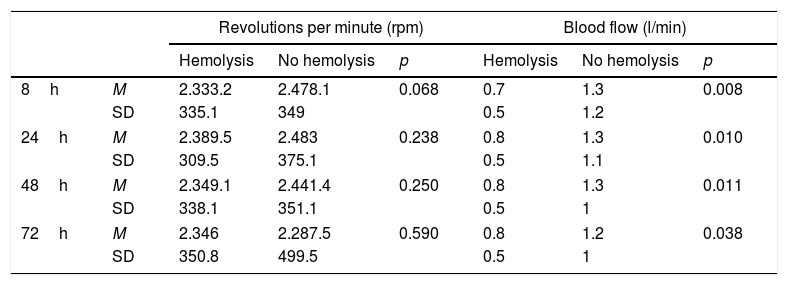
No relationship was observed between hemolysis and the number of revolutions used ECMO. Blood flow in the children with hemolysis was lower than in those without hemolysis (Table 4).
Relationship between hemolysis and the number of revolutions and flow of the circuit.
| Revolutions per minute (rpm) | Blood flow (l/min) | ||||||
|---|---|---|---|---|---|---|---|
| Hemolysis | No hemolysis | p | Hemolysis | No hemolysis | p | ||
| 8h | M | 2.333.2 | 2.478.1 | 0.068 | 0.7 | 1.3 | 0.008 |
| SD | 335.1 | 349 | 0.5 | 1.2 | |||
| 24h | M | 2.389.5 | 2.483 | 0.238 | 0.8 | 1.3 | 0.010 |
| SD | 309.5 | 375.1 | 0.5 | 1.1 | |||
| 48h | M | 2.349.1 | 2.441.4 | 0.250 | 0.8 | 1.3 | 0.011 |
| SD | 338.1 | 351.1 | 0.5 | 1 | |||
| 72h | M | 2.346 | 2.287.5 | 0.590 | 0.8 | 1.2 | 0.038 |
| SD | 350.8 | 499.5 | 0.5 | 1 | |||
SD: standard deviation; ECMO: extracorporeal membrane oxygenation; h: hours; M: mean.
Extracorporeal membrane oxygenation could be suspended in 76 patients (due to clinical improvement in 61 cases, bleeding in one, conversion to a ventricular assist system in 7, and heart transplantation in 7). The duration of ECMO was 5.5 days (IQR 3–9 days). In total, 52 patients were still alive at discharge from hospital.
The duration of admission to the PICU was 21 days (IQR 10–38 days). There were no significant differences in the duration of admission to the PICU between the patients with initial bleeding (40.2 days; SD 25.1) and the rest (49.6 days; SD 44) (p=0.385).
Forty-eight percent of the children died: 56% of those under one year of age and 40% of those over this age (p=0.161). Mortality among the children with bleeding at the start of ECMO (57.6%) was significantly greater than in the rest of the patients (37.5%) (p=0.048).
Mortality among the children with coagulation disorders was greater than in the rest, though the differences were not statistically significant: INR<2 (63.2% versus 44.4%; p=0.202), fibrinogen<150mg/dl (72.7% versus 44.9%; p=0.112) and <100,000platelets/mm3 (56.7% versus 44.3%; p=0.282).
Lastly, mortality among the patients who were in the postoperative period of heart surgery (53.1%) was greater than in the rest (39%), though the difference failed to reach statistical significance (p=0.213).
DiscussionThe results of our study show that hematological alterations are very frequent in children subjected to ECMO, and are associated to important transfusion requirements.
The incidence of bleeding in our study was higher than that reported in the ELSO registry,2,4 though we included any type of bleeding and the percentage of postsurgery patients was very high. This could partially account for the observed differences. In the present study the coagulation disorders and transfusion requirements remained stable in the two periods of the study.
Some authors have suggested that only bleeding requiring transfusion should be regarded as a bleeding complication. Other investigators propose classification of bleeding into mild (between 10 and 20ml/kg in 24h) or intense (>20ml/kg in 24h, with a drop in hemoglobin of 2g/l in 24h, involvement of the brain, lungs or retroperitoneum, or a need for surgery).12 Bleeding volume was not recorded in our study, but 39% of the patients required revision surgery.
The cause of bleeding in patients with ECMO is probably multifactorial.2 On one hand, the underlying disease, surgery, drug use and multiorgan failure usually present in these children alter the coagulation mechanisms. On the other hand, the ECMO machine and system produce inflammation, platelet activation1,8,13 and the consumption of coagulation factors.3 Lastly, treatment with heparin is another cause of bleeding.
An important decrease in platelet count is observed in children and adults subjected to ECMO, and frequent transfusions are required.1,13–15 The etiology underlying thrombocytopenia is probably multifactorial (infection, bleeding, transfusion and platelet depletion in the ECMO circuit).8 We recorded a significant decrease in platelet count (from 186,500 to 105,700/mm3) in the first 24h of treatment. This figure persisted in the course of treatment, with periodic platelet transfusions.
Bleeding, platelet activation and the consumption of coagulation factors explain the hematological alterations found in these patients and the need for blood product transfusions. In our study, the factors associated to an increased need for blood product transfusions were the postoperative period of heart surgery, bleeding at the start of ECMO, and the need for revision surgery. Other authors have reported similar findings.16,17 In the logistic regression analysis, the factors found to be significantly correlated to the need for packed red cell transfusions were the impossibility of suspending extracorporeal circulation and transthoracic cannulation.
There is no clear evidence regarding the indications of transfusions in children subjected to ECMO.12 Most authors propose keeping the hematocrit at 35%, with a broad transfusion indication range of between 25 and 40%, and the platelet count at 100,000/mm3, with a transfusion indication range of between 50,000 and 200,000/mm3.12 These transfusion criteria are similar to those used in our series. Further studies are needed to determine whether a more restrictive transfusion policy could reduce the volume of transfusions without placing the patient at risk.
Heparin response is highly variable and changing over time, and it is difficult to keep the circuit free of thrombi without increasing the risk of bleeding.18 A number of studies have reported a greater correlation between heparin levels and antiXa than with ACT or aPTT,8,19 and the antiXa levels moreover are not affected by acute phase reactants such as fibrinogen and factor vii.8 Other studies have reported a lesser need for blood sampling, a decrease in blood product transfusions, and fewer both thrombotic and bleeding complications when using antiXa as part of the coagulation monitoring protocol.19
In our study we used ACT for monitoring anticoagulation at the patient bedside, and aPTT and antiXa for the daily control of anticoagulation. Our reference parameter for the control of anticoagulation was antiXa. However, since this parameter cannot be determined on an emergency basis in our hospital, use was made of ACT for the hourly regulation of heparin dosing, and aPTT was used to assess the need for plasma transfusion. The antiXa levels initially were maintained below the therapeutic range in most of the patients with respect to the use of lower heparin doses to reduce bleeding.
Some investigators have reported that the use of lower heparin doses for keeping aPTT between 1.5 and 2 times the control level results in a lesser incidence of bleeding without incrementing thromboembolic events16 though large comparative trials are needed to confirm these results.
Thromboembolic events occur as a consequence of contact between the blood and non-endothelial surfaces, triggering an inflammatory response with the activation of coagulation. In our study, 14 patients suffered some thromboembolic event, with peripheral thrombi being the most frequent presentation. The 6 patients that presented these events died under conditions of multiorgan failure probably related to disseminated intravascular coagulation (DIC). In the ELSO registry, the central nervous system was the most commonly affected territory, with 5% of the children suffering cerebral infarction.4 This is consistent with our own findings (3%).
Thrombi in the oxygenator were recorded in 21 patients – this being the most common location of thrombosis within the circuit.3,6,20 The figure is similar to those reported by other authors.3,20 Thrombi on the arterial side of the oxygenator are the most important risk factor for thromboembolism. In this regard, careful and continuous circuit monitoring is required by the medical and nursing staff, with close control of anticoagulation. The appearance of thrombi in the post-oxygenator circuit requires circuit replacement in order to avoid embolisms. This situation occurred in 10 of our patients.
In our study, 33% of the patients presented hemolysis – this figure being significantly lower than the 66% reported by Lou et al.21
Hemolysis is a complication of mechanical support secondary to increased shear stress caused by the pump and which can lead to acute renal failure and multiorgan failure.3 The negative pressure drop across the oxygenator membrane can also produce a suction effect with cavitation and red cell damage. On the other hand, the use of high revolution settings has also been related to hemolysis; as a result, when free hemoglobin starts to rise in blood, it is advisable where possible to reduce the revolutions per minute (rpm).3 In children with hemolysis, Lou et al. found the rpm setting and flow to be greater than in patients without hemolysis.21 However, no relationship between rpm and hemolysis was observed in our study, and surprisingly the blood flow was lower in the children with hemolysis than in those without.
On the other hand, in our series hemolysis was greater in the children of younger age and lower weight, in coincidence with the observations of Lou et al.21
The survival rate at hospital discharge in our study (52%) was very similar to that reported by the ELSO registry in cardiac pediatric patients (51%).4
Bleeding is the main cause of mortality in patients subjected to ECMO.3,6 In our case, mortality among the children with bleeding at the start of ECMO (57.6%) was significantly greater than in the rest of the patients (37.5%), in coincidence with the findings of the ELSO registry.2,6 Major bleeding can contribute to the appearance of organic complications and increases coagulopathy, generating a vicious circle of coagulopathy-bleeding-transfusions that proves very difficult to control.
The median duration of ECMO support in our study (5.5 days) was similar to that reported in the ELSO registry among children with cardiac indications for extracorporeal support (6.3 days).4
The median duration of stay in the PICU among the patients requiring ECMO was 21 days, and the presence of bleeding was not associated to longer admission.
Our study has a number of limitations. Firstly, it is a single-center trial conducted in a heart surgery reference center, and our data are not fully extrapolatable to other centers in which ECMO is used in other types of patients. Secondly, the study involves a retrospective analysis of a registry with a long duration – a fact that increases the difficulty of adequately collecting all the variables and the risk of bias; full homogeneity of the transfusion criteria cannot be ensured, and causal relationships cannot be established.
Lastly, bleeding volume was not quantified and the hematological parameters were influenced by the number of transfusions received by the patients. In our study we did not record the values of the hematological parameters before and after each transfusion, which would have been of help in assessing the impact of the transfusions.
Our results show hematological and coagulation disorders to be frequent in the children subjected to ECMO, with a high incidence of bleeding and the need for numerous transfusions.
The patients in the postoperative period of surgery, those with bleeding at the start of ECMO, and those needing revision surgery required a greater volume of transfusions – though no causal relationship can be established in this regard. The children with bleeding at the start of ECMO showed higher mortality but not a longer PICU stay. The present study may serve as a basis for the development of specific transfusion protocols in children subjected to ECMO and for the design of multicenter studies seeking to determine whether heparin-impregnated circuits, lesser anticoagulation, and a more restricted transfusion policy are able to reduce the risk of bleeding and thromboembolic complications in children subjected to ECMO.19
Financial supportThe authors have received no financial support for carrying out this study.
Authorship/collaborationsMJS: conception and design of the study; data collection, analysis and interpretation; critical review of the text; and approval of the definitive version of the manuscript.
CG: data collection, analysis and interpretation; drafting of the text; and approval of the definitive version of the manuscript.
IM: data collection, analysis and interpretation; drafting of the text; and approval of the definitive version of the manuscript.
VM: data collection, analysis and interpretation; drafting of the text; and approval of the definitive version of the manuscript.
PS: data collection, analysis and interpretation; drafting of the text; and approval of the definitive version of the manuscript.
AS: conception and design of the study; data collection, analysis and interpretation; critical review of the text; and approval of the definitive version of the manuscript.
JLH: conception and design of the study; analysis and interpretation of the data; drafting of the text; and approval of the definitive version of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors are grateful to the medical and nursing staff of the PICU, the Pediatric Cardiology Section and Pediatric Heart Surgery, the perfusion nurses, and the Department of Hematology of Hospital General Universitario Gregorio Marañón, for their collaboration in the care of the children subjected to ECMO.
Please cite this article as: Santiago MJ, Gómez C, Magaña I, Muñoz V, Saiz P, Sánchez A, et al. Complicaciones hematológicas en niños tratados con oxigenación por membrana extracorpórea. Med Intensiva. 2019;43:281–289.