To study the results and complications of endovascular treatment (EVT) in acute ischemic stroke patients admitted to intensive care unit (ICU). To analyse the possible factors related to mortality and level of disability at ICU discharge and one year after stroke.
DesignObservational prospective study.
SettingMixed ICU. Third level hospital.
PatientsSixty adult patients. Consecutive sample.
InterventionsNone.
Variables of interestEpidemiological data, time from symptom onset to EVT, angiographic result, length of stay, days on mechanical ventilation, neurological complications, National Institutes of Health Stroke Scale (NIHSS) at ICU admission and discharge, modified Rankin scale score (mRS) at one year.
ResultsMean age 68.90±8.84 years. Median time from symptom onset to EVT: 180min. Median NIHSS at admission: 17.5; at discharge: 3. Distal flow was achieved in 90% of cases. Median ICU stay: 3 days. Mechanical ventilation: 81.7%. Functional independence (mRS≤2) 50% at one year. Deaths: 22 (36,6%) of which 8 (13.3%) died during UCI stay and the rest during the first year.
ConclusionsThe factors relating to a worse functional outcome were symptomatic hemorrhage transformation, lack of recanalization and complications during EVT. The factors relating to mortality were symptomatic hemorrhage and hydrocephalus. Distal flow was achieve in most cases with a low complication rate. Half of the patients presented functional independence one year after the stroke.
Estudiar los resultados y las complicaciones del tratamiento endovascular (TEV) en pacientes con ictus isquémico agudo ingresados en una unidad de cuidados intensivos (UCI). Analizar los factores que podrían influir en la mortalidad y en el grado de discapacidad al alta y un año después del ictus.
DiseñoEstudio prospectivo observacional.
ÁmbitoUCI polivalente. Hospital de tercer nivel.
PacientesSesenta pacientes adultos. Muestra consecutiva.
IntervencionesNinguna.
Variables de interésDatos epidemiológicos, tiempo desde la clínica inicial hasta el TEV, resultado angiográfico, tiempo de estancia en UCI, días de ventilación mecánica, complicaciones neurológicas, National Institutes of Health Stroke Scale (NIHSS) al ingreso y al alta de UCI, escala de Rankin modificada (mRS) al año de evolución.
ResultadosEdad media 68,90 ± 8,84 años. Mediana de tiempo hasta el TEV: 180min. Mediana NIHSS al ingreso: 17,5; al alta: 3. Flujo distal en el 90% de los casos. Mediana estancia en UCI: 3 días. Ventilación mecánica: 81,7%. Independencia funcional (mRS ≤ 2) 50% al año del ictus. Fallecimientos: 22 (36,6%); 8 (13,3%) en la UCI y el resto durante el primer año.
ConclusionesLas variables asociadas a un peor estado funcional fueron la transformación hemorrágica sintomática, la ausencia de recanalización y las complicaciones durante el procedimiento. La transformación hemorrágica y la hidrocefalia se asociaron a mayor mortalidad. Se consiguió flujo distal en la mayoría de los casos, con una baja tasa de complicaciones. La mitad de los pacientes alcanza independencia funcional al año del ictus.
In developed countries, ischemic stroke is one of the leading causes of disability and the second most common cause of death. The disease has a devastating effect upon patient quality of life, with extremely high healthcare costs, and constitutes a very important public health problem.1
Over 85% of all cases of stroke are of an ischemic nature – fundamentally of cardioembolic or atherothrombotic origin.2 Arterial occlusion results in diminished cerebral blood flow (CBF). When this flow drops to under 10ml/100g/min, cell death and infarction occur. Between the infarct zone and the brain tissue with normal CBF (50ml/100g/min) lies a hypoperfused zone of variable extent in which two areas can be distinguished: an oligohemic area with a CBF of over 22ml/100g/min that rarely evolves toward infarction, and an ischemic penumbra area with a CBF of under 22ml/100g/min that progresses toward cerebral infarction if perfusion is not quickly restored. The main objective of treatment in cases of stroke is to secure urgent recanalization of the obstructed artery, with early reperfusion of the brain tissue.3 This concept is embodied within the term “time is brain”. Subsequent patient management in the stroke unit or intensive care unit (ICU) is essential to obtain better functional outcomes.4,5
Thrombolysis with intravenous recombinant tissue plasminogen activator (IV r-tPA) has been shown to be effective and safe since publication of the National Institute of Neurological Disorders and Stroke (NINDS) trial.6 Following the results of the European Cooperative Acute Stroke Study (ECASS-3), the use of IV r-tPA has been extended to cases of ischemic stroke with an evolution of up to 4.5h.7 The limitations of the treatment are a narrow therapeutic window, lesser efficacy in application to large vessel occlusions and thrombi measuring over 8mm in size,8 and contraindication in patients with a high risk of bleeding complications.5
In addition to treatment with IV r-tPA, there are endovascular reperfusion techniques that achieve higher recanalization rates by acting at the site of the lesion, and which allow the treatment of a larger number of patients thanks to a wider therapeutic window.9
A number of recent studies have demonstrated the superiority of mechanical thrombectomy versus treatment exclusively based on IV r-tPA in patients with large vessel occlusion and an evolution of up to 8h. Endovascular treatment (EVT) improves the functional outcomes without increasing mortality.10–14
The present study was carried out to analyze the results and complications of EVT with or without IV r-tPA therapy in patients with acute ischemic stroke admitted to the ICU.
Patients and methodsStudy designA prospective observational study was carried out in a university hospital serving as reference center in Neurosurgery and Interventional Neuroradiology. We included all patients with acute ischemic stroke subjected to mechanical thrombectomy and admitted to the ICU between June 2012 and February 2014. Admission to the ICU was decided according to the criterion of the neurologist, based on the need for ventilation support, poor neurological condition after the procedure and/or complications related to EVT. The study was approved by the Research Ethics Committee of the Principado de Asturias (Spain)(reference no. 148/15).
Study variables- -
Demographic: age and gender.
- -
Clinical: history of cardiovascular risk factors and previous stroke, and the National Institutes of Health Stroke Scale (NIHSS) score upon hospital admission.
- -
Related to the procedure: time to EVT, location of the obstruction, indication of treatment, previous IV r-tPA use, endovascular techniques employed, recanalization and complications.
- -
Neurological complications during admission to the ICU: hemorrhagic transformation, hydrocephalus, seizures. Systemic complications: respiratory problems (pneumonia, tracheobronchitis, atelectasis, acute lung edema), cardiogenic shock. Requirements in the ICU: ventilatory support, need for vasoactive drugs.
- -
Outcome variables: functional assessment and mortality at discharge from the ICU, mortality during the first year of follow-up, and degree of disability one year after the ischemic event.
Upon admission, the patients were evaluated by a neurologist. Computed tomography (CT) of the brain, CT angiography (CTA) and perfusion CT were performed to assess brain tissue viability, selecting those patients amenable to derive greater clinical benefit. Cerebral arteriography was performed to locate the vascular occlusion site and assess collaterality. The femoral artery was the access route used. Endovascular treatment was carried out by expert neuroradiologists under general anesthesia or sedation, depending on the clinical condition of the patient and the criterion of the anesthetist. Patients with a low level of consciousness or offering no collaboration underwent sedoanalgesia with mechanical ventilation. Following the procedure, the patients were admitted to the ICU.
TreatmentOf the 207 patients with acute ischemic stroke subjected to EVT in our center, the 60 subjects (29%) meeting criteria of clinical severity were admitted to the ICU. The inclusion criteria for EVT are specified in Table 1. Patients were required to meet all these criteria in order to be included in the study. The indication of intravenous fibrinolytic treatment was based on the criterion of the neurologist.
Endovascular treatment inclusion criteria.
| Age>18 years |
| Time from symptoms onset to arrival in hospital: |
| <6h in anterior circulation |
| <12h in posterior circulation |
| NIHSS≥7 |
| Brain CT without hemorrhage or infarction |
| CTA with large vessel occlusion or contraindication/failure of systemic fibrinolysis |
| Perfusion CT: brain tissue viability (discrepancy>20%) |
CTA: CT angiography; NIHSS: National Institutes of Health Stroke Scale; CT: computed tomography.
Mechanical extraction of the thrombus was carried out using a self-expanding and extractable Solitaire FR® stent (Covidien-ev3Inc, Irvine, CA, USA). The technique consists of advancing a microguide (Concentric Medical Inc., CA, USA) and microcatheter with a Rapidtransit® balloon (Codman & Shurtleff, Inc., MA, USA) until the obstruction site is traversed. The device is then expanded and compresses the thrombus against the wall of the vessel to secure recanalization. The balloon of the guide catheter is insufflated, the stent is withdrawn with continuous aspiration to avoid embolisms, and the thrombus is extracted simultaneously with the stent. In the percutaneous transluminal angioplasty procedures we used UltraSoft® balloons (Stryker Neurovascular, Fremont, CA, USA). The stents placed corresponded to the Wingspan® Stent System in intracranial position and the carotid Wallstent® (both from Boston Scientific, MA, USA).
Arterial recanalization was scored by means of the arterial occlusive lesion (AOL) scale: 0=no recanalization; 1=partial or incomplete recanalization of the occlusion without distal flow; 2=partial or incomplete recanalization of the occlusion with distal flow; and 3=complete recanalization with distal flow.15 We defined treatment failure as AOL 0–1 and efficacy as AOL 2–3.
Evaluation of the resultsSymptomatic cerebral hemorrhage was defined according to the ECASS-II criteria as evidence of cerebral hemorrhage in any part of the brain, associated to neurological deterioration with an increase of at least four points on the NIHSS.16
The effect of treatment at discharge from the ICU was documented based on the NIHSS in all survivors. Functional assessment was based on the modified Rankin scale (mRS),17 with good functional status being defined by mRS≤2. Functional evaluation after one year was based on the data obtained from the clinical review made by the Department of Neurology, and through telephone conversation.
Statistical analysisA descriptive analysis of the results was carried out using the SPSS version 19.0 statistical package for MS Windows (SPSS Inc., Chicago, IL, USA).
Continuous variables were reported as the mean and standard deviation (SD) in the presence of a normal sample distribution as confirmed by the Kolmogorov–Smirnov test. The median and interquartile range (IQR) were calculated in the presence of a non-normal distribution, in the case of time variables, or when the SD exceeded the mean. Categorical variables in turn were reported as absolute and relative frequencies.
The comparison of continuous variables (considering that only two independent samples were compared) was based on the Student t-test for independent samples in the presence of a normal distribution, and using the nonparametric Mann–Whitney U-test in the case of a non-normal distribution.
The analysis of a given variable at different timepoints was carried out using the Student t-test for paired samples or the Wilcoxon signed-rank test in the case of a non-normal frequency distribution.
The independence of categorical variables was contrasted using the Chi-squared test, with use of the Fisher exact test in those cases where the conditions for application of the Chi-squared test were not met.
The Mantel–Haenszel odds ratio (ORMH) was used as risk measure, with inclusion of the respective 95% confidence interval (CI).
Statistical significance was considered for p<0.05.
ResultsClinical characteristicsThe mean patient age was 68.90±8.84 years (males 67.12±8.26; females 73.41±8.89). Males predominated: 43 (71.7%). There was no statistically significant association between age and mortality or functional status either at discharge or after one year. Likewise, there were no gender differences in relation to the clinical outcomes.
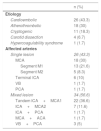
Most of the patients (83.3%) had at least one cardiovascular risk factor: arterial hypertension (n=36; 60%); diabetes mellitus (n=13; 21.7%); dyslipidemia (n=23; 38.3%); smoking, 11 (18.3%). Six patients (10%) had suffered previous stroke. The etiology of stroke is reported in Table 2.
Etiology of stroke and location of the lesion.
| n (%) | |
|---|---|
| Etiology | |
| Cardioembolic | 26 (43.3) |
| Atherothrombotic | 18 (30) |
| Cryptogenic | 11 (18.3) |
| Carotid dissection | 4 (6.7) |
| Hypercoagulability syndrome | 1 (1.7) |
| Affected arteries | |
| Single lesion | 26 (43.3) |
| MCA | 18 (30) |
| Segment M1 | 13 (21.6) |
| Segment M2 | 5 (8.3) |
| Terminal ICA | 6 (10) |
| VB | 1 (1.7) |
| PCA | 1 (1.7) |
| Mixed lesion | 34 (56.6) |
| Tandem ICA+MCA1 | 22 (36.6) |
| ICA+MCA2 | 7 (11.6) |
| ICA+PCA | 1 (1.7) |
| MCA+ACA | 1 (1.7) |
| VB+PCA | 3 (5) |
ACA: anterior cerebral artery; ICA: internal carotid artery; MCA: middle cerebral artery; MCA1: segment M1 of the middle cerebral artery; MCA2: segment M2 of the middle cerebral artery; PCA: posterior cerebral artery; VB: vertebrobasilar artery.
The median time from the onset of symptoms to the start of EVT was 180min (interquartile range: 131.25–237.50). There were no statistically significant differences in functional status or mortality according to the time elapsed to the start of treatment.
National Institutes of Health Stroke ScaleThe median NIHSS score upon admission was 17.5 points (interquartile range: 14–20). The NIHSS score could not be determined in two patients admitted in deep coma. There were no statistically significant differences in NIHSS score upon admission between the patients that died and those that survived. However, higher NIHSS scores upon admission were associated to poorer functional status at discharge from the ICU and after one year (p=0.027 and p=0.036, respectively). The median NIHSS score upon admission among the patients with favorable outcomes one year after stroke was 16.5 points (interquartile range: 13–19).
Evaluation of the effect of treatmentThe median NIHSS score at the time of discharge from the ICU was 3 points (interquartile range: 1.25–11.50). Statistically significant differences were observed on comparing the NIHSS score upon admission versus the score at discharge (p<0.01). The median decrease in NIHSS score at discharge was 11 points (interquartile range: 6–15).
Indication of treatmentThe indication of EVT was established as a result of failure of fibrinolysis with IV r-tPA in 8 patients (13.3%); the contraindication of fibrinolytic treatment in 8 patients (13.3%); and the location of large vessel obstruction in 44 subjects (73.3%). Of these, 7 patients (11.6%) received incomplete treatment with alteplase until the endovascular procedure was available. In relation to this latter indication, we observed an association between the administration of IV r-tPA and good functional status at discharge from the ICU (p=0.014), as well as survival after one year (p=0.037).
Location of the lesion, endovascular treatment and complications of the techniqueThe location of arterial occlusion is reported in Table 2. A total of 56.6% of the patients had two or more affected arteries. There was no statistically significant relationship between any of the obstruction sites and mortality or functional status. Internal carotid artery-middle cerebral artery (ICA-MCA) tandem lesions were likewise not related to poorer functional status or increased mortality risk.
The technique employed, the results and the complications are reported in Table 3.
Endovascular techniques and their complications.
| n (%) | Deaths, n | mRS≥3, n | |
|---|---|---|---|
| Technique | |||
| Mechanical thrombectomy | 60 (100) | ||
| Stent | |||
| Extracranial | 15 (25) | ||
| + Angioplasty | 1 (1.7) | ||
| Intracranial | 6 (10) | ||
| + Angioplasty | 2 (3.3) | ||
| Intracranial stent+extracranialstent+angioplasty | 1 (1.7) | ||
| Angioplasty | 1 (1.7) | ||
| Recanalization | |||
| Complete (AOL 3) | 41 (68.3) | 5 | 14 |
| Incomplete (AOL 2) | 13 (21.7) | 0 | 5 |
| Failed (AOL 0 and 1) | 6 (10) | 3 | 6 |
| Complications during the technique | |||
| Distal embolism | 9 (15) | 1 | 4 |
| Arterial dissection | 3 (5) | 0 | 1 |
| Arterial rupture | 1 (1.7) | 1 | 1 |
| Stent stenosis | 1 (1.7) | 0 | 0 |
| Detachment of the device | 1 (1.7) | 1 | 1 |
AOL: arterial occlusive lesion.
Of the 54 patients subjected to effective recanalization treatment (AOL 2 and 3), 35 showed good functional status at discharge (64.9%). In contrast, of the 6 patients in which recanalization did not prove effective (AOL 0 and 1), none presented good functional status at discharge (p<0.01). After one year, 30 of the patients with effective treatment maintained good functional status (55.6%), while all patients in the failed therapy group showed poor functional status (p=0.024).
One patient suffered arterial perforation during the treatment and died in the ICU. Other serious complications were detachment of the thrombus extracting device in a patient that likewise died during admission, and stent stenosis in a patient that died after 5 months. The patients that suffered complications during the procedure had a 3.7-fold lesser probability of presenting good functional status after one year than the patients that did not suffer complications (p=0.019; ORMH 0.27 [95%CI: 0.1–0.8]).
Stay in the intensive care unitThe median stay in the ICU was days (interquartile range: 2–5.75).
Complications in the intensive care unitThe requirements and complications in the ICU are summarized in Table 4.
Complications and requirements in the intensive care unit.
| n (%) | Deaths, n | mRS≥3, n | |
|---|---|---|---|
| Neurological complications | |||
| Symptomatic hemorrhagic transformation | 8 (13.3) | 3 | 8 |
| Hydrocephalus | 4 (6.7) | 3 | 4 |
| Seizures | 2 (3.3) | 0 | 1 |
| Systemic complications | |||
| Pneumonia | 5 (8.3) | 2 | 3 |
| Tracheobronchitis | 10 (16.7) | 2 | 8 |
| Atelectasis | 5 (8.3) | 1 | 3 |
| Cardiogenic shock | 1 (1.7) | 1 | 1 |
| Acute lung edema | 4 (6.7) | 2 | 2 |
| Others | 5 (8.3) | 1 | 5 |
| Complications at the puncture site | |||
| Pseudoaneurysm | 1 (1.7) | 0 | 0 |
| Local bleeding | 2 (3.3) | 0 | 0 |
| Femoral thrombosis | 1 (1.7) | 0 | 1 |
| Requirements ICU | |||
| Mechanical ventilation | 49 (81.7) | 8 | 24 |
| Vasoactive drugs | 23 (38.3) | 8 | 14 |
Of the 8 patients with symptomatic hemorrhagic transformation, three died in the ICU (37.5%), versus 5 of the 52 patients without this complication (9.6%) (p=0.031). After one year, 7 of the patients with hemorrhagic transformation had died (87.5%), versus 15 of those without hemorrhagic transformation (28.8%) (p<0.01). A statistically significant correlation to functional status at discharge was also recorded (p<0.01). Specifically, none of the patients with hemorrhagic transformation presented good functional status after one year, versus 57.7% of the patients that did not suffer this complication (p<0.01).
Of the four patients with hydrocephalus, none survived the first year after stroke, while 38 of the 56 patients without hydrocephalus survived (67.9%) (p=0.015). One of the patients required external ventricular drainage of cerebrospinal fluid.
Systemic complicationsOf the 40 patients with systemic complications, 8 (20%) died in the ICU, while the 20 patients without such complications all survived (p=0.043). Furthermore, of the 40 patients with systemic complications, 21 had poor functional status at discharge (55%), versus four of the 20 patients without systemic complications (20%) (p=0.016).
Of the 10 patients that developed tracheobronchitis, 8 presented poor functional status at discharge (80%), versus 17 of the 50 patients without tracheobronchitis (34%) (p=0.012). After one year, the same number of patients that presented tracheobronchitis continued to show poor functional status, versus 22 of the 50 patients without tracheobronchitis (44%) (p=0.038).
Other complications comprised acute renal failure, urinary tract infection (two cases), bacteremia and upper digestive bleeding.
Treatment in the intensive care unitThe median duration of mechanical ventilation was 7.5h (interquartile range: 2–41). Of the 49 patients that required invasive ventilatory support, 24 presented poor functional status at discharge (48%), versus a single subject among the 11 patients that did not require such support (9.1%) (p=0.019).
Of the 23 patients requiring vasoactive drugs, 8 died during admission (34.8%), versus none of the 37 patients that did not require such medication (p<0.01). At discharge from the ICU, 14 of the patients that required vasoactive drugs showed poor functional status (60.9%). In comparison, 11 of the patients that did not require vasoactive drugs showed poor functional status (29.7%) (p=0.017).
Following EVT, 39 patients received antiplatelet medication (65%) and 9 received anticoagulants (15%).
Clinical courseWith regard to mortality, 8 patients died in the ICU (13.3%) – in two cases due to causes unrelated to stroke (cardiogenic shock and acute renal failure with acute lung edema). The stroke-related causes of death were intracranial hypertension syndrome secondary to cerebral infarction (4 patients), associated to hemorrhagic transformation in two cases, and limitation of therapeutic effort (due to a lack of neurological recovery) in two cases. Another 14 patients died during the year of follow-up (23.3%) – in 5 cases due to causes unrelated to stroke (acute myocardial infarction, pancreatic tumor, respiratory failure in two cases, and infectious complications in one patient).
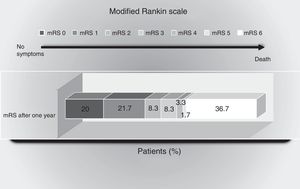
With regard to functional status, during the year of follow-up 30 patients showed a favorable course, with mRS≤2 (50%). The distribution of functional course is shown in Fig. 1.
Scores of the modified Rankin scale (mRS) one year after the ischemic event. The percentage of patients with scores between 0 and 6 on the mRS are shown: 0=no symptoms; 1=no major disability (able to carry out routine activities and duties); 2=mild disability (unable to carry out some previous activities, but able to care for own interests and concerns without help); 3=moderate disability (symptoms that significantly restrict lifestyle or impede full autonomy: some help required); 4=moderately severe disability (symptoms that clearly impede independent living, though without the need for continuous care: unable to attend personal needs without help); 5=severe disability (totally dependent: needs constant attention, day and night); 6=death.
Our results show the factors associated to increased patient disability to be the absence of recanalization, complications during the endovascular procedure, and symptomatic hemorrhagic transformation. The presence of systemic complications, the need for vasoactive drugs, ventilatory support, and the development of tracheobronchitis also imply poorer functional status at discharge from the ICU, though this is probably because of the clinical condition and complexity of the patients in the ICU, associated to increased resource utilization compared with other types of patients. The variables related to mortality were seen to be hemorrhagic transformation and hydrocephalus. Table 5 summarizes the variables associated to poorer functional outcomes and mortality. The incidence of symptomatic cerebral hemorrhage recorded in our series (13.3%) is greater than that reported in other studies. This is probably due to both the definition used (the criteria of the Safe Implementation of Thrombolysis in Stroke Monitoring Study [SITS-MOST] being much more restrictive than those of the ECASS-IV and to the greater severity of the patients.
Summary of the variables associated to poorer functional outcomes and mortality.
| Variables | Comparison factors | |||
|---|---|---|---|---|
| Death at discharge | Functional status at discharge | Death after one year | Functional status after one year | |
| Absence of recanalization | p<0.01 | p=0.024 | ||
| Complications during the procedure | p=0.019 | |||
| Symptomatic hemorrhagic transformation | p=0.031 | p<0.01 | p<0.01 | p<0.01 |
| Hydrocephalus | p=0.015 | |||
| Complications systemic | p=0.043 | p=0.016 | ||
| Tracheobronchitis | p=0.012 | p=0.038 | ||
| Need for vasoactive drugs | p<0.01 | p=0.017 | ||
| Need for mechanical ventilation | p=0.019 | |||
In the management of acute ischemic stroke, the accepted first therapeutic option is IV r-tPA, though its narrow therapeutic window, the poor results obtained in cases of large vessel obstruction, and the strict patient eligibility criteria all limit its use. As a result, EVT with or without intravenous fibrinolytic therapy, is an important alternative, since it affords good results in the first 6h from symptoms onset.10–14 Endovenous treatment secures rapid restoration of CBF, is safe, affords improved functional outcomes, and shows a global tendency toward reduced patient mortality versus conventional treatment.18 These outcomes are more favorable in cases involving a shorter evolution from symptoms onset. This is causing some clinicians in routine practice to obviate intravenous fibrinolytic therapy or limit its dosage in an attempt to minimize the treatment times. Furthermore, certain thrombus locations such as the terminal internal carotid artery (ICA), the proximal middle cerebral artery (MCA) or tandem lesions (ICA-MCA), have shown poorer recanalization rates with IV r-tPA,19 and these were precisely the most frequent locations in our study.
The latest clinical trials10–14 have studied recanalization in the 6 first hours (except the REVASCAT study, which contemplated a limit of 8h), in patients with large vessel occlusion and the use of stent-retrievers. The main differences of our study are the lesser percentage of patients receiving previous treatment with IV r-tPA; the greater severity as evidenced by a higher NIHSS score and the fact that the clinical instability of the patients precluded their admission to the Stroke Unit; and the assessment of functional status after one year instead of follow-up during 90 days after the ischemic event.
In our study, the time from symptoms onset to EVT (median 180min) was shorter than reported in the literature (between 185 and 269min).10–14 No statistically significant difference was observed in the clinical course as a function of time. The fact that earlier treatment was provided in our series possibly could have masked statistically significant differences that would have been observed had the time periods been longer. The need for early treatment in order to restore CBF would justify the use of any strategy allowing sooner EVT—in our case elimination or incomplete treatment with IV r-tPA until the endovascular became available. Therefore, one of the differences of our study is the low percentage of patients previously treated with IV r-tPA. In this regard, one of our limitations is that we eliminated the benefits which combination treatment could have provided.
In our series, all the procedures were carried out with Solitaire FR®, and distal flow was achieved in 90% of the cases—this percentage being higher than in other studies (thrombolysis in cerebral infarction [TICI] 2b/3 of 59% in MR CLEAN, 72% in ESCAPE, 86% in EXTEND-IA, 88% in SWIFT PRIME and 66% in REVASCAT).18 Our results confirm the relationship between recanalization and good functional status. The low complications rate observed is in close agreement with the data obtained in the aforementioned clinical trials.
Most of the studies assessed functional status after 90 days, while in our series follow-up was continued up to one year after stroke. Our functional independence rate after one year was 50%, versus 33% in MR CLEAN, 44% in REVASCAT, 53% in ESCAPE, 60% in SWIFT-PRIME and 71% in EXTEND-IA10–14 analyzed after 90 days.
The NIHSS remains confirmed as a good prognostic indicator. The clinical benefit of the treatment was independent of patient age, and although ours was a rather elderly series, the survival rate after one year was 63.3%. Table 6 compares the results of the main clinical trials and our own.
Demographic data and results of the recent randomized and controlled studies on endovascular treatment. Comparison with the results of the present study.
| Studies | Mean age | Basal NIHSS (median) | Treatment with IV r-tPA (%) | TICI 2b/3 (%) | Time to puncture (median) | Cervical ICA stenosis/occlusion | General anesthesia (%) | mRS 0–2 after 90 days (%) |
|---|---|---|---|---|---|---|---|---|
| MR CLEAN | 65.8 | 17 | 87 | 59 | 260 | 13 (stent) | 38 | 33 |
| ESCAPE | 71 | 16 | 73 | 72 | 185 | 13 | 9 | 53 |
| EXTEND-IA | 68.6 | 17 | 100 | 86 | 210 | 0 | 36 | 71 |
| SWIFT PRIME | 65 | 17 | 100 | 88 | 224 | 0 | 37 | 60 |
| REVASCAT | 65.7 | 17 | 68 | 66 | 269 | 8.7 (stent) | 7 | 44 |
| Present results | 68.9 | 17.5 | 25 | 90a | 180 | 15 (stent) | 81.7 | 50b |
ICA: internal carotid artery; IV r-tPA: intravenous recombinant tissue plasminogen activator; mRS: modified Rankin scale; NIHSS: National Institutes of Health Stroke Scale; TICI: thrombolysis in cerebral infarction.
The possible strengths of our study are its setting (the ICU) and the one-year follow-up period—this being the longest period reported to date in the literature. With regard to the limitations of our study, mention must be made of patient selection bias—since we only included patients admitted to the ICU (and who presumably are in a more serious condition)—the limited sample size, and the lack of a control group.
The use of EVT can restore CBF in patients not amenable to IV r-tPA or in those in which the latter treatment has failed. Mechanical thrombectomy with stent-retriever devices affords good outcomes in the hands of multidisciplinary teams and in specialized centers,20 though some aspects remain to be clarified despite the existing evidence. Criteria must be established for predicting which patients can benefit from such treatment, in order to minimize the risks and costs of the process.
The limitations of mechanical thrombus removal include its limited availability, since specialized centers are required to offer such treatment, and its increased technical complexity. Furthermore, it must be emphasized that recanalization and a good angiographic outcome are not always associated to greater clinical benefit—the latter depending on many factors, such as the location/size of the thrombus, the clinical severity of the patient, and the time elapsed from symptoms onset.
There is still some negative discrimination in the management of acute stroke, with a clear disadvantage in terms of infrastructure and human resources, when compared with coronary disease. Considering that stroke is one of the leading causes of mortality and disability, its treatment poses an important challenge for the healthcare system, with the need for implementation and an increased availability of neurointerventional teams. The high price involved in terms of mortality and patient quality of life makes the addressing of this issue an inexcusable ethical and professional obligation.
Financial supportThe present study has received no financial support.
AuthorshipLucía Viña-Soria, Dolores Escudero-Augusto and Lorena Martín-Iglesias conceived and designed the study.
Lucía Viña-Soria, Dolores Escudero-Augusto, Lorena Martín-Iglesias, Lucía López-Amor, Iván Astola-Hidalgo, Lorena Forcelledo-Espina, Raquel Rodríguez-García, Sara de Cima-Iglesias, José Antonio Gonzalo-Guerra, Eduardo Murias-Quintana, Pedro Vega-Valdés and Sergio Calleja-Puerta contributed to data acquisition, analysis and interpretation, and to critical review of the manuscript.
All the authors have approved the presented version of the article.
Conflicts of interestNone declared.
Thanks are due to Josefina Alonso-Fernandez (Hospital Universitario Central de Asturias) for her contribution to data processing and statistical analysis.
Please cite this article as: Viña Soria L, Martín Iglesias L, López Amor L, Astola Hidalgo I, Rodríguez García R, Forcelledo Espina L, et al. Resultados y evolución funcional de pacientes críticos con ictus isquémico sometidos a trombectomía mecánica. Med Intensiva. 2018;42:274–282.