The aim of this study was to establish the incidence of septic cardiomyopathy (SM) in patients with sepsis and septic shock, to describe its characteristics and testing its evolution.
DesignProspective cohort study.
ParticipantsWe included 57 consecutive patients admitted to Intensive Care Unit, who met criteria of sepsis and septic shock.
Principal variables of interestClinical and biochemical variables were analyzed. An echocardiogram was performed in the first 24h of admission, determining myocardial function parameters, and if the patients had left ventricular ejection fraction (LVEF) <50%) a second echocardiogram was performed.
AmbitIntensive medical and surgical Care Service for Adults in University Hospital.
ResultsThe mean age of the patients was 62.1±16.3 years. 58% were males. 22.8% had left ventricular dysfunction. The mean LVEF in patients with MS was lower than those without SM (34.1±10.6 vs 60.7±6.94%, P<.001), with complete recovery, in survivors, after the acute event (LVEF at discharge 56.1±6.3%, P=.04). Patients with SM had higher levels of procalcitonin (47.1±35.4 vs 18.9±24.5; P=.02) and higher score on the Sequential Organ Failure Assessment (SOFA score) (9.91±3.82 vs 7.47±3.41; P=.037). Mortality was not significantly different between both groups [4 (30.8%) vs 4 (9.1%); P=.07].
ConclusionsSM is not uncommon and is related to a higher scores on the severity scales. In the survivors, LVEF normalized after the recovery of the acute event.
El objetivo de este estudio fue determinar la incidencia de miocardiopatía séptica (MS) en pacientes con sepsis y shock séptico, describir sus características y comprobar su evolución.
DiseñoEstudio prospectivo sobre una cohorte.
ParticipantesSe incluyeron 57 pacientes consecutivos ingresados en Unidad de Cuidados Intensivos, con criterios de sepsis y shock séptico.
Variables de interés principalesSe analizaron variables clínicas y bioquímicas. Se realizó un ecocardiograma en las primeras 24h de ingreso, determinando parámetros de función cardiaca, y si los pacientes presentaban una fracción de eyección de ventrículo izquierdo (FEVI)<50%, se realizó un ecocardiograma evolutivo.
ÁmbitoServicio de Medicina Intensiva médico-quirúrgico de adultos en Hospital Universitario.
ResultadosLa edad media de los pacientes fue de 62,1±16,3 años, el 58% fueron varones. El 22,8% presentaron disfunción de ventrículo izquierdo. La FEVI media en los pacientes con MS fue inferior respecto a los que no la tenían (34,1±10,6 vs. 60,7±6,94%; p<0,001), con recuperación completa de la misma, en los supervivientes, tras el evento agudo (FEVI al alta 56,1±6,3%; p=0,04). Los pacientes con MS, presentaban mayores niveles de procalcitonina (47,1±35,4 vs. 18,9±24,5; p=0,02) y puntuación en la escala Sequential Organ Failure Assessment (SOFA) (9,91±3,82 vs. 7,47±3,41; p=0,037). La mortalidad no fue significativamente diferente entre ambos grupos (4 [30,8%] vs. 4 [9,1%]; p=0,07).
ConclusionesLa MS no es infrecuente, se relaciona con mayor puntuación en las escalas de gravedad. En los supervivientes, la FEVI se normalizó tras la recuperación del evento agudo.
Sepsis is defined as potentially fatal organ dysfunction caused by an inadequate host response to infection. Organ dysfunction in turn corresponds to an acute change of two points on the Sequential Organ Failure Assessment (SOFA) scale, as a consequence of infection.1 Myocardial involvement in this scenario, known as septic cardiomyopathy (SM) in its most common presentation, is characterized by left ventricular systolic myocardial dysfunction. It was first described by Parker in 1984 as a decrease in ventricular ejection fraction with an increase in end-diastolic volume in septic shock patients.2
The underlying physiopathological mechanisms comprise a series of systemic factors generated by an inflammatory state mediated by interleukins, tumor necrosis factor, myocyte contraction failure and mitochondrial dysfunction, among others.3,4 Septic cardiomyopathy is a transient condition that resolves within 7–10 days after onset,5 though in some cases the disorder can persist for a longer period of time. Improved knowledge of this disease is of interest, in view of its potential implications for the prognosis and treatment of septic patients.
Different studies on SM can be found in the literature, with different conclusions, different inclusion criteria, and no clear agreement regarding the incidence of the disease or its differential characteristics. Transthoracic echocardiography is typically used for the diagnosis of SM.6 The present study seeks to determine the incidence of SM in a sample of septic patients in our setting, using transthoracic ultrasound, and to describe the characteristics and evolution of the affected patients.
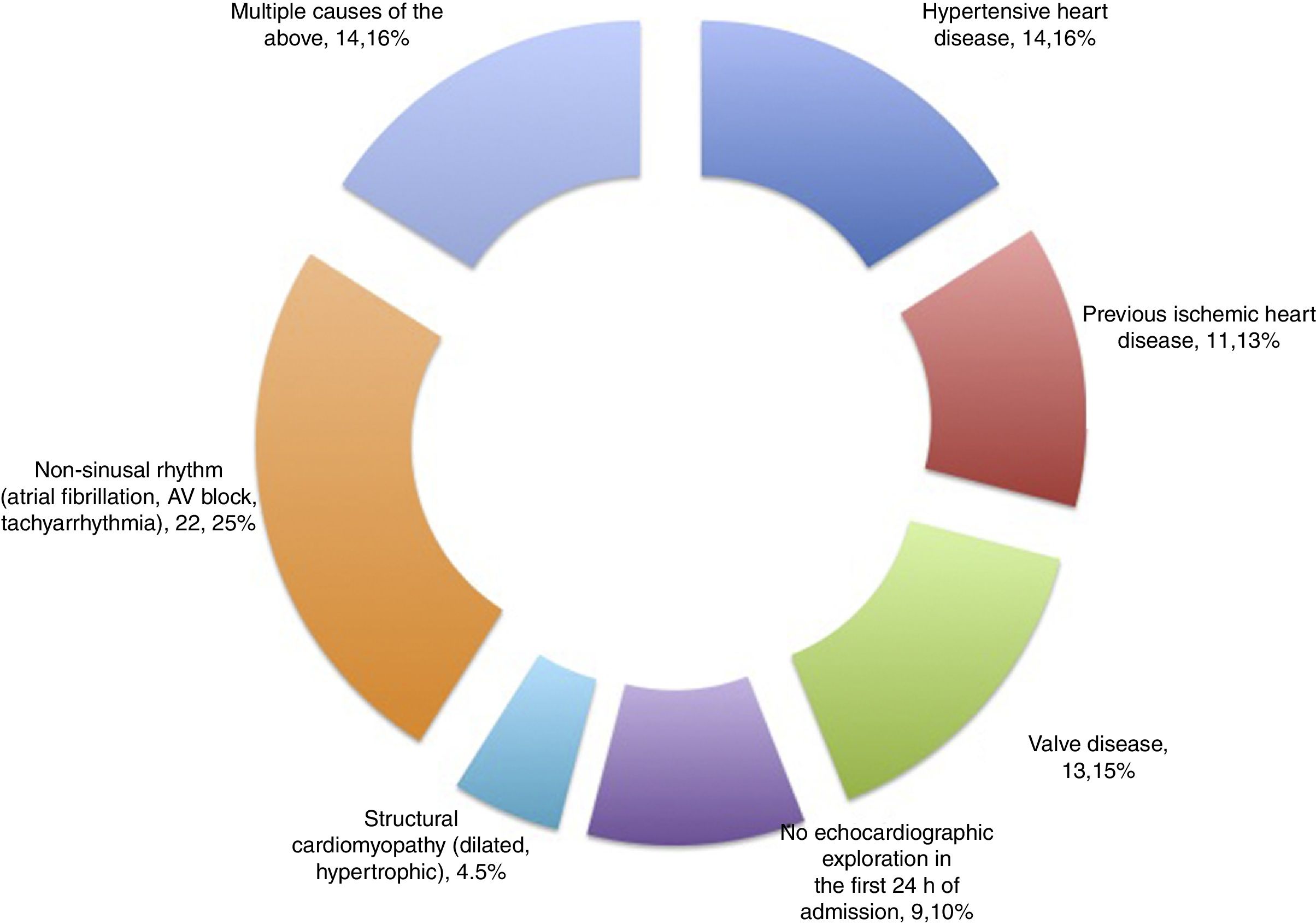
Patients and methodsA prospective cohort study was carried out, with the consecutive inclusion of all patients meeting criteria of sepsis and septic shock admitted during the period between May 2014 and October 2015 to a polyvalent, 26-bed adult Intensive Care Unit (ICU) serving a population of 475,958 inhabitants. The patients presented predominantly medical and neurological disease, and surgical cases comprised neurosurgical and maxillofacial surgery patients, as well as polytrauma cases. An annual average of 110 patients with sepsis and septic shock are admitted to the Unit. We excluded all patients with a history of heart disease (based on clinical diagnosis or detection from the echocardiographic history), including hypertensive heart disease, valve disorders, prior ischemia and/or acute coronary syndrome, as well as the absence of sinus rhythm (fibrillation, atrial flutter or tachyarrhythmia, any type of atrioventricular block, or the presence of some cardiac electrostimulation device), structural cardiomyopathy (dilated, hypertrophic), combinations of the above, and the lack of protocolized echocardiographic exploration in the first 24h following admission.
Data were compiled related to the patient history (arterial hypertension, diabetes mellitus, dyslipidemia, smoking, alcoholism, obesity [body mass index ≥30kg/m2], peripheral vascular disease, ischemic or hemorrhagic stroke, chronic renal failure [defined as serum creatinine >1.5mg/dl] and chronic liver damage [defined as any grade of cirrhosis or chronic disease accompanied by any grade of known liver failure]), type of patient (medical or surgical), infection site, causal pathogens and evolutive information (stay in ICU, post-ICU stay, and death), and the Acute Physiology and Chronic Health Evaluation (APACHE II) and SOFA scores. Biochemical parameters were recorded upon admission and every 8h, documenting peak concentration in the first 24h corresponding to C-reactive protein, procalcitonin, NT-ProBNP, lactate, creatinine and bilirubin. A blood count was obtained, together with a coagulation study, and the need for intravenous dobutamine and noradrenalin was determined. The patients were subjected to invasive monitoring (Pulse Induced Contour Cardiac Output [PICCO®]) according to the supervising physician. Measurements were obtained of cardiac index, global end-diastolic volume and extravascular lung water.7
The evaluated echocardiographic parameters (obtained in the first 24h and at hospital discharge) were the end-diastolic diameter of the left ventricle (LV), left ventricular ejection fraction (LVEF) determined by the Simpson 4C method and through visual estimation, the left ventricular outflow tract velocity-time integral (LVOT VTI), the E/E′ wave ratio (E′ being regarded as the septal and lateral average), the degree of mitral valve insufficiency, tricuspid annular plane systolic excursion (TAPSE),8 and the lateral tricuspid annulus tissue Doppler S wave. The echocardiographic evaluation was carried out in the first 24h of patient admission by a member of the Department of Cardiology who recorded the study for posterior analysis by another independent cardiologist. A third evaluation was made with the recorded images in the event of discrepancies between the two interpretations.
Septic cardiomyopathy was defined as LV systolic dysfunction, taken to correspond to LVEF <50%, attributable to sepsis (excluding patients with previous heart disease), associated or not to right ventricle (RV) systolic dysfunction or LV diastolic dysfunction (understood as an alteration of the normal E/A wave ratio of the mitral filling pattern [E/A ratio <0.8; E/A >2], as well as E/E′ ratio >15).9
Lactate concentration and central venous saturation were evaluated in order to assess a possible association to the presence or absence of SM.
The study was approved by the Research Ethics Committee of our center on 28 March 2014. The obtainment of written informed consent was not considered necessary.
Statistical analysisQuantitative variables were reported as the mean and standard deviation (SD), while categorical variables were reported as absolute number and percentage. The chi-squared test was used for the comparison of categorical variables, with application of the Fisher test when the conditions for chi-squared testing were not met in the 2×2 tables. Pairs of quantitative variables in turn were compared using the Student t-test or Mann–Whitney U-test, depending on the distribution of the variables. The Student t-test for paired samples was used to assess significance of a given variable in one same individual (e.g., evolution of LVEF upon admission and at hospital discharge). Statistical significance was considered for p<0.05.
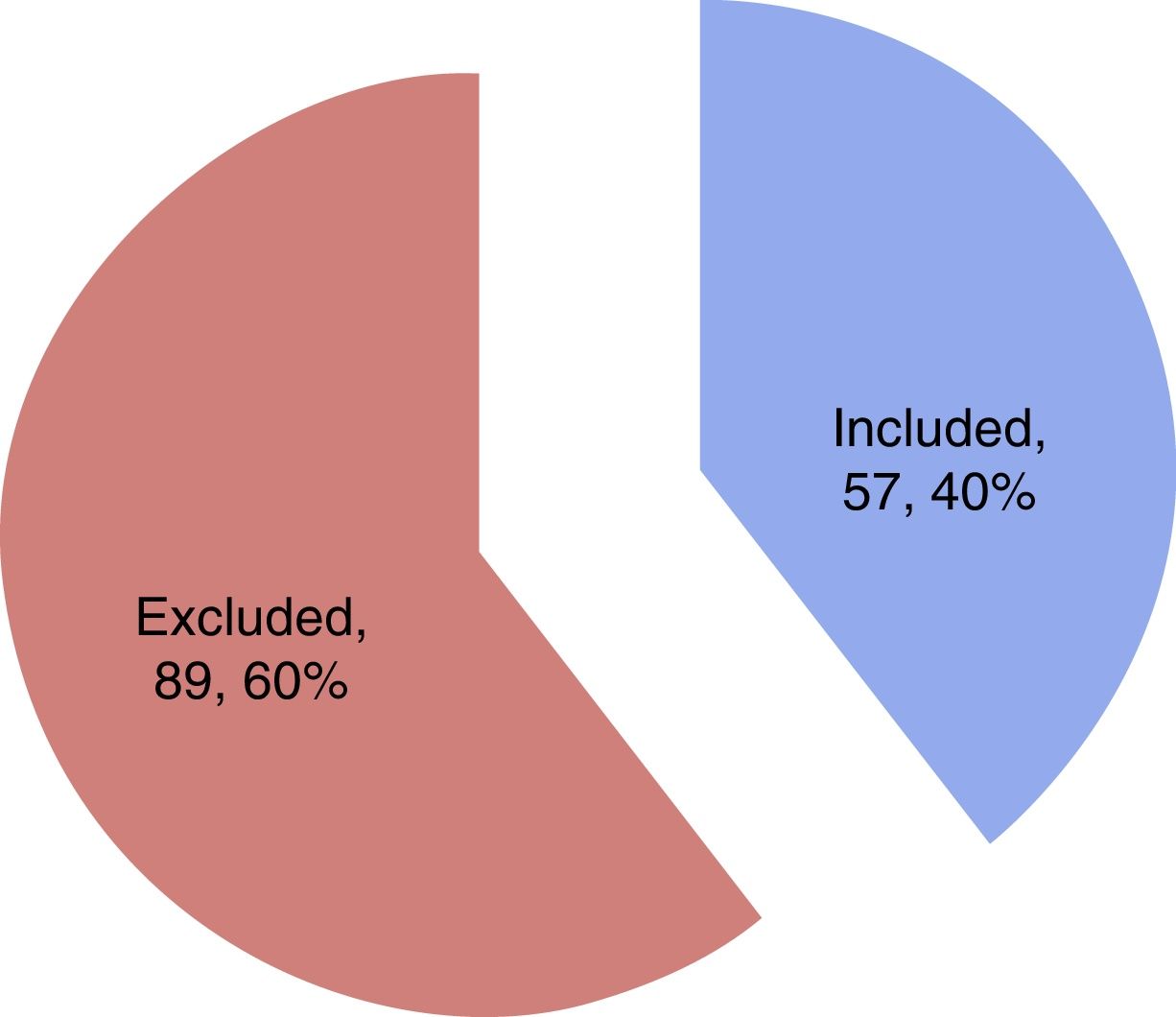
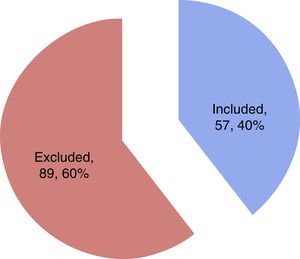
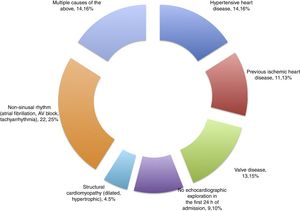
ResultsA total of 57 patients were included in the study. Thirteen subjects (22.8%) presented SM in the first 24h as diagnosed from the echocardiographic study. The mean patient age was 62.1±16.3 years, and 57.9% were males. Table 1 details the baseline characteristics and personal history. During the study period, a total of 144 patients with sepsis and/or septic shock were admitted to the ICU, of which 57 were included in the study while 87 were excluded for the reasons indicated in Fig. 1.
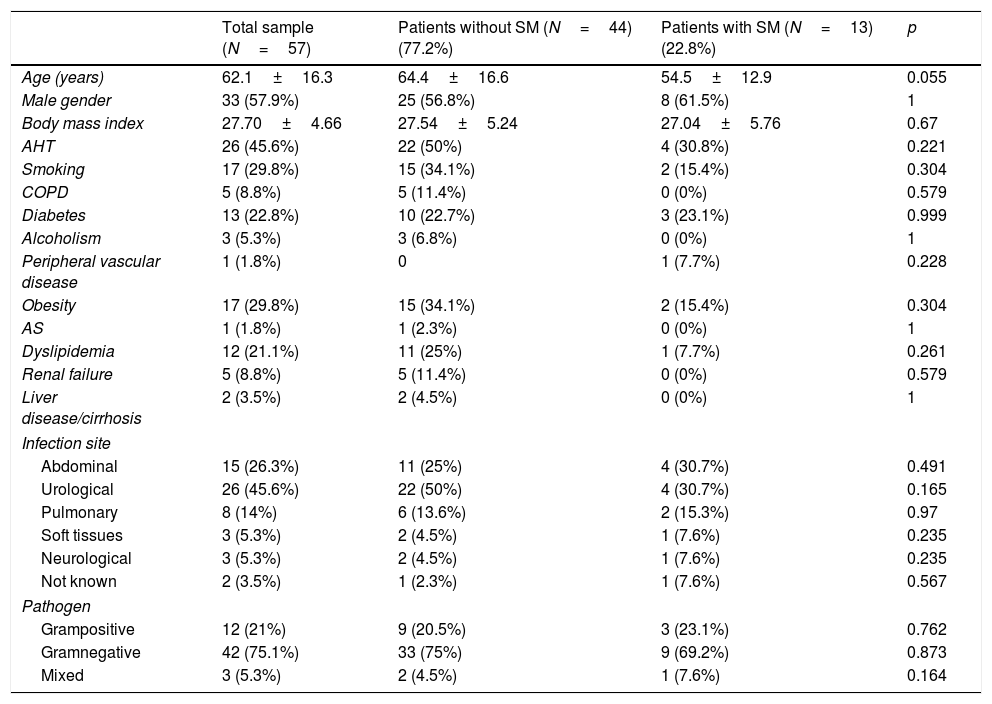
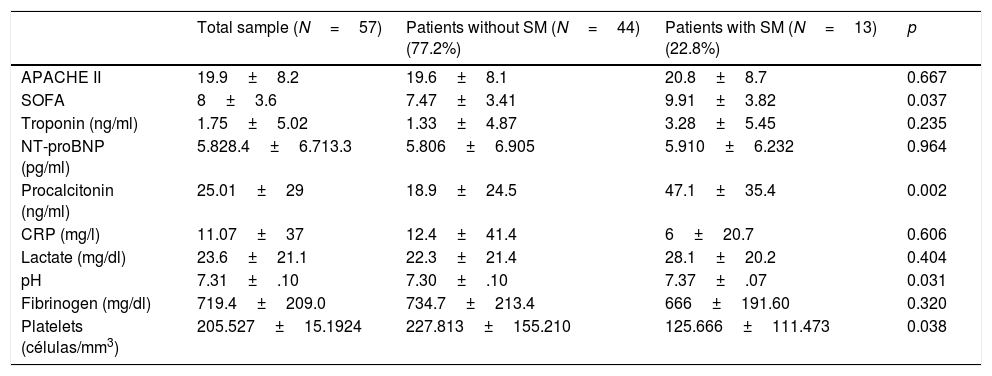
Baseline characteristics, personal history, infection site and type of pathogen in the total patients and in those with and without septic cardiomyopathy.
| Total sample (N=57) | Patients without SM (N=44) (77.2%) | Patients with SM (N=13) (22.8%) | p | |
|---|---|---|---|---|
| Age (years) | 62.1±16.3 | 64.4±16.6 | 54.5±12.9 | 0.055 |
| Male gender | 33 (57.9%) | 25 (56.8%) | 8 (61.5%) | 1 |
| Body mass index | 27.70±4.66 | 27.54±5.24 | 27.04±5.76 | 0.67 |
| AHT | 26 (45.6%) | 22 (50%) | 4 (30.8%) | 0.221 |
| Smoking | 17 (29.8%) | 15 (34.1%) | 2 (15.4%) | 0.304 |
| COPD | 5 (8.8%) | 5 (11.4%) | 0 (0%) | 0.579 |
| Diabetes | 13 (22.8%) | 10 (22.7%) | 3 (23.1%) | 0.999 |
| Alcoholism | 3 (5.3%) | 3 (6.8%) | 0 (0%) | 1 |
| Peripheral vascular disease | 1 (1.8%) | 0 | 1 (7.7%) | 0.228 |
| Obesity | 17 (29.8%) | 15 (34.1%) | 2 (15.4%) | 0.304 |
| AS | 1 (1.8%) | 1 (2.3%) | 0 (0%) | 1 |
| Dyslipidemia | 12 (21.1%) | 11 (25%) | 1 (7.7%) | 0.261 |
| Renal failure | 5 (8.8%) | 5 (11.4%) | 0 (0%) | 0.579 |
| Liver disease/cirrhosis | 2 (3.5%) | 2 (4.5%) | 0 (0%) | 1 |
| Infection site | ||||
| Abdominal | 15 (26.3%) | 11 (25%) | 4 (30.7%) | 0.491 |
| Urological | 26 (45.6%) | 22 (50%) | 4 (30.7%) | 0.165 |
| Pulmonary | 8 (14%) | 6 (13.6%) | 2 (15.3%) | 0.97 |
| Soft tissues | 3 (5.3%) | 2 (4.5%) | 1 (7.6%) | 0.235 |
| Neurological | 3 (5.3%) | 2 (4.5%) | 1 (7.6%) | 0.235 |
| Not known | 2 (3.5%) | 1 (2.3%) | 1 (7.6%) | 0.567 |
| Pathogen | ||||
| Grampositive | 12 (21%) | 9 (20.5%) | 3 (23.1%) | 0.762 |
| Gramnegative | 42 (75.1%) | 33 (75%) | 9 (69.2%) | 0.873 |
| Mixed | 3 (5.3%) | 2 (4.5%) | 1 (7.6%) | 0.164 |
AS: acute stroke; COPD: chronic obstructive pulmonary disease; AHT: arterial hypertension; SM: septic cardiomyopathy.
Quantitative variables expressed as mean±standard deviation.
Most of the patients (68.4%) came from the emergency service, 24.6% from the hospital ward, and 7% were transferred from another hospital. The patients were predominantly medical cases (83.3%), while 8.3% were surgical patients and 8.3% polytrauma cases. The main infection site corresponded to the urological tract, and gramnegative bacteria were the most frequent organisms causing sepsis. There were no significant differences between the site of infection or the type of pathogen in the compared groups (Table 1).
Table 2 shows the findings considered globally and according to groups with or without SM, referred to the prognostic scales and laboratory test determinations of interest, documenting the most altered value in the first 24h of admission. Significantly higher procalcitonin levels were recorded in the patients that developed SM (47.1±35.4 vs. 18.9±24.5; p=0.002). Furthermore, the patients with SM yielded higher scores on the prognostic and multiorgan dysfunction scales such as the SOFA (9.91±3.82 vs. 7.47±3.41; p=0.037) and APACHE II (20.8±8.7 vs. 19.6±8.1; p=0.667) – though in this latter case statistical significance was not reached.
Prognostic scales and laboratory test determinations of interest in the total patients and in the groups with or without septic cardiomyopathy.
| Total sample (N=57) | Patients without SM (N=44) (77.2%) | Patients with SM (N=13) (22.8%) | p | |
|---|---|---|---|---|
| APACHE II | 19.9±8.2 | 19.6±8.1 | 20.8±8.7 | 0.667 |
| SOFA | 8±3.6 | 7.47±3.41 | 9.91±3.82 | 0.037 |
| Troponin (ng/ml) | 1.75±5.02 | 1.33±4.87 | 3.28±5.45 | 0.235 |
| NT-proBNP (pg/ml) | 5.828.4±6.713.3 | 5.806±6.905 | 5.910±6.232 | 0.964 |
| Procalcitonin (ng/ml) | 25.01±29 | 18.9±24.5 | 47.1±35.4 | 0.002 |
| CRP (mg/l) | 11.07±37 | 12.4±41.4 | 6±20.7 | 0.606 |
| Lactate (mg/dl) | 23.6±21.1 | 22.3±21.4 | 28.1±20.2 | 0.404 |
| pH | 7.31±.10 | 7.30±.10 | 7.37±.07 | 0.031 |
| Fibrinogen (mg/dl) | 719.4±209.0 | 734.7±213.4 | 666±191.60 | 0.320 |
| Platelets (células/mm3) | 205.527±15.1924 | 227.813±155.210 | 125.666±111.473 | 0.038 |
APACHE II: Acute Physiology and Chronic Health Evaluation II; SM: septic cardiomyopathy; NT-proBNP: N-terminal brain natriuretic peptide; CRP: C-reactive protein; SOFA: Sequential Organ Failure Assessment.
The laboratory test parameters correspond to the most altered values recorded in the first 24h, coinciding with the first echocardiographic exploration.
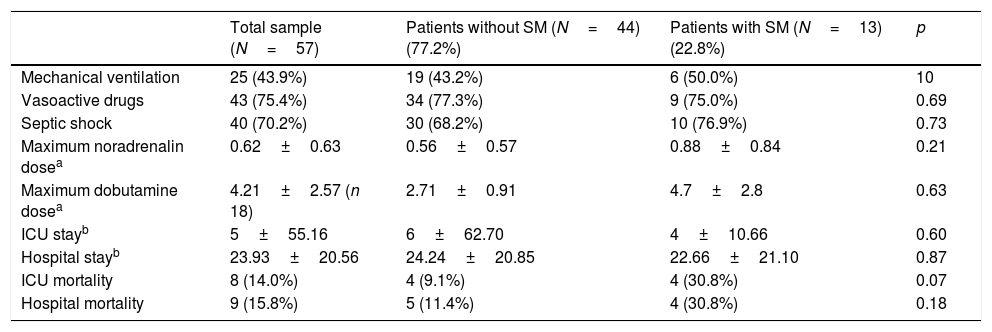
Table 3 shows the general evolutive data. The patients that died did so during admission to the ICU. Of note is the fact that the mortality rate was greater in the group of patients with SM (30.8%, 4 patients) than in the group without SM (9.1%, 4 patients, p=0.07) – though statistical significance was not reached.
Data of interest during follow-up in the ICU and in hospital referred to the total patients and groups with and without septic cardiomyopathy.
| Total sample (N=57) | Patients without SM (N=44) (77.2%) | Patients with SM (N=13) (22.8%) | p | |
|---|---|---|---|---|
| Mechanical ventilation | 25 (43.9%) | 19 (43.2%) | 6 (50.0%) | 10 |
| Vasoactive drugs | 43 (75.4%) | 34 (77.3%) | 9 (75.0%) | 0.69 |
| Septic shock | 40 (70.2%) | 30 (68.2%) | 10 (76.9%) | 0.73 |
| Maximum noradrenalin dosea | 0.62±0.63 | 0.56±0.57 | 0.88±0.84 | 0.21 |
| Maximum dobutamine dosea | 4.21±2.57 (n 18) | 2.71±0.91 | 4.7±2.8 | 0.63 |
| ICU stayb | 5±55.16 | 6±62.70 | 4±10.66 | 0.60 |
| Hospital stayb | 23.93±20.56 | 24.24±20.85 | 22.66±21.10 | 0.87 |
| ICU mortality | 8 (14.0%) | 4 (9.1%) | 4 (30.8%) | 0.07 |
| Hospital mortality | 9 (15.8%) | 5 (11.4%) | 4 (30.8%) | 0.18 |
SM: septic cardiomyopathy; ICU: Intensive Care Unit.
The following data were obtained in the patients subjected to PICCO® monitoring (40 in total: 11 with SM and 29 without SM): cardiac index 1.2±0.3l/min/m2 vs. 3.66±1.50l/min/m2 in the patients with and without SM, respectively (p=0.058); global end-diastolic volume 481.5±74.2ml/m2 vs. 754.1±212.5ml/m2 (p=0.124); and extravascular lung water 6.5±2.12 vs. 9.8±2.03 (p=0.070) – the differences between the two groups being nonsignificant. No significant differences were observed with regard to vasoactive drug treatment or the required noradrenalin (0.88±0.84 vs. 0.56±0.57μg/kg/min; p=0.211) and dobutamine doses (4.7±2.8 vs. 2.71±0.91μg/kg/min; p=0.63) in patients with SM, taking into account that noradrenalin was administered in 40 patients and dobutamine in 18.
Forty patients suffered septic shock (70.2%), with no significant correlation to the presence or absence of SM, since three patients with systolic dysfunction did not present hemodynamic shock. In relation to the above, we observed no significant differences between the two groups in terms of lactate concentration (23.03±21.68 vs. 27.59±19.54; p=0.51) or central venous saturation (74.69±13.34 vs. 68.32±16.25; p=0.40).
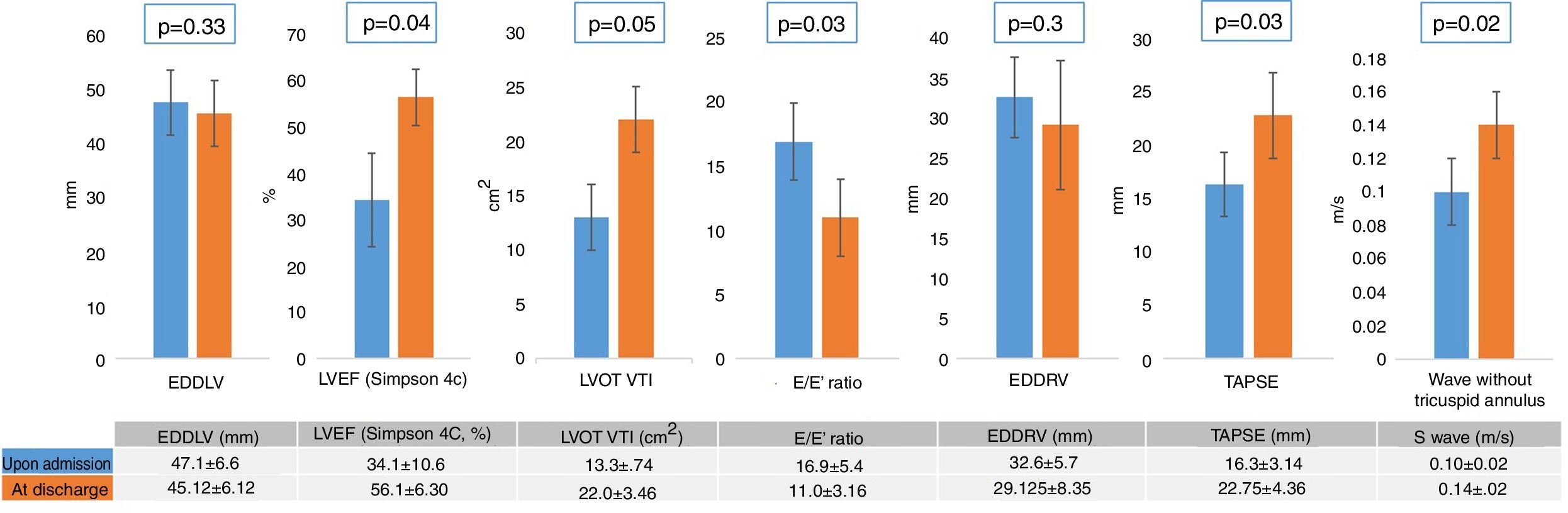
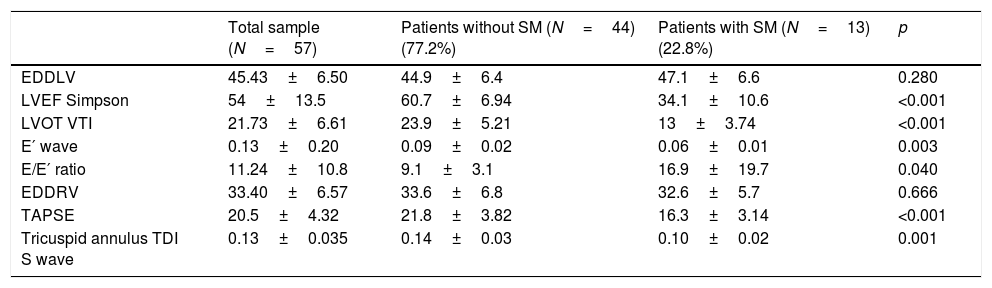
With regard to the echocardiographic data (Table 4), we recorded no significant differences in LV end-diastolic diameter between the patients with and without SM. On the other hand, the patients with SM showed an increase in left cavity filling pressure as a marker of diastolic dysfunction, reflected by the E/E′ ratio (p=0.046), as well as RV systolic dysfunction, measured by the lateral tricuspid annulus tissue Doppler S wave (0.14±0.03 vs. 0.10±0.02; p=0.001) and TAPSE (21.8±3.82 vs. 16.3±3.14; p<0.001). The control echocardiogram showed reversion of LV and RV dysfunction (Fig. 2) in all survivors with SM (Fig. 3).
Echocardiographic variables upon admission referred to the total patients and groups without and with septic cardiomyopathy.
| Total sample (N=57) | Patients without SM (N=44) (77.2%) | Patients with SM (N=13) (22.8%) | p | |
|---|---|---|---|---|
| EDDLV | 45.43±6.50 | 44.9±6.4 | 47.1±6.6 | 0.280 |
| LVEF Simpson | 54±13.5 | 60.7±6.94 | 34.1±10.6 | <0.001 |
| LVOT VTI | 21.73±6.61 | 23.9±5.21 | 13±3.74 | <0.001 |
| E′ wave | 0.13±0.20 | 0.09±0.02 | 0.06±0.01 | 0.003 |
| E/E′ ratio | 11.24±10.8 | 9.1±3.1 | 16.9±19.7 | 0.040 |
| EDDRV | 33.40±6.57 | 33.6±6.8 | 32.6±5.7 | 0.666 |
| TAPSE | 20.5±4.32 | 21.8±3.82 | 16.3±3.14 | <0.001 |
| Tricuspid annulus TDI S wave | 0.13±0.035 | 0.14±0.03 | 0.10±0.02 | 0.001 |
EDDRV: end-diastolic diameter of the right ventricle; EDDLV: end-diastolic diameter of the left ventricle; TDI: tissue Doppler; LVEF: left ventricular ejection fraction; LVOT VTI: left ventricular outflow tract velocity-time integral; SM: septic cardiomyopathy; TAPSE: tricuspid annular plane systolic excursion.
Variation of the echocardiographic measurements. EDDRV: end-diastolic diameter of the right ventricle; EDDLV: end-diastolic diameter of the left ventricle; LVEF: left ventricular ejection fraction; LVOT VTI: left ventricular outflow tract velocity-time integral; TAPSE: tricuspid annular plane systolic excursion.
There is some controversy regarding the clinical importance of SM, its impact upon patient mortality, and the most reliable parameters for assessing the disorder. Some authors10 consider that the degree of physiological coherence of the indexes most commonly used to measure heart function is currently not clear. In our study the criterion for diagnosing myocardial dysfunction related to sepsis and septic shock has been LVEF <50%, as indicated in the Mayo Clinic study conducted by Pulido et al.6 – though it is true that other investigators apply lower LVEF cut-off points, such as Vieillard-Baron,11 who uses the definition LVEF <40% and a cardiac index of <3l/min/m2. Although the first publications on SM estimated a prevalence of 60%,12 the most recent studies set the prevalence between 26 and 27.7%.6,13 This means that the prevalence recorded in our study (22.8%) lies at the lower limit of the most recently published values.6,14 This may be due to several factors. On one hand, we applied strict inclusion and exclusion criteria, while on the other the evaluation of SM was made in the first 24h of patient admission – in contrast to other authors who evaluate patients for SM during the first 2–3 days of admission.6,10,11,15 Furthermore, in our study the definition of SM was restricted to systolic dysfunction, without including LV diastolic dysfunction and RV dysfunction (considered isolatedly or combined) as done by other investigators. For example, in the study published by Pulido et al.,6 involving one of the largest patient samples to date, the incidence of SM was 64% (68 of the 106 patients included in the study developed the disorder). The authors defined SM as the presence of any of the following: LV systolic dysfunction, LV diastolic dysfunction or RV systolic dysfunction. However, on analyzing those patients with isolated LV systolic dysfunction, the incidence decreased to 27% (29 patients), which is more consistent with the figure recorded in our own series. Likewise, the high incidence of diastolic dysfunction in the elderly population, with comorbidities, and in critically ill patients,16 makes it very difficult to demonstrate that the disorder can be attributed to sepsis. This is the reason why diastolic dysfunction was excluded in the present study.17 It also should be commented that late myocardial dysfunction18 occurs from 24h of admission and particularly when LV postload normalizes. This type of dysfunction was not studied in our sample in order to better differentiate intervention of the patient inflammatory state and the physiopathology of sepsis and its resolution. On considering this form of late dysfunction, the global prevalence of SM increases, as has been described in some studies such as that published by Boissier et al.,10 with a cardiac dysfunction rate of 22% in the first hours of admission that is similar to the figure recorded in our series – though the rate was seen to increase to 31.8% on including the cases detected on the second and third day. A relationship was also observed in this study between mortality and the cases presenting a hyperkinetic pattern with low postload – this contradicting the findings of other studies that relate myocardial systolic dysfunction to increased patient mortality.
With regard to the baseline condition of the patients, and in coincidence with other studies, we found no differences between the groups.6,19 On the other hand, the patients that developed SM were comparatively younger – the difference coming close to statistical significance. The older age of patients who do not develop SM has been noted in other studies,16 though some authors have reported the opposite, i.e., septic dysfunction being observed in older individuals.6 The result obtained in our series may be due to the fact that we excluded patients with cardiac comorbidity, which are generally older individuals.
As to whether the origin of sepsis can influence the development of SM, we found no association, as commented above. The main infection site causing sepsis in our patient cohort corresponded to the urological tract, since this presentation involved lesser associated cardiac comorbidity (which constituted an exclusion criterion). Some studies have attempted to establish a causal relationship between lung infections and SM, though the acute respiratory distress syndrome that typically accompanies such infections often results in a degree of right ventricle failure.20
The patients with SM yielded a significantly higher SOFA score than those who did not develop SM. This was also noted in the study of Pulido et al.,6 and would reflect the presence of SM within the context of a greater number of failing organs. Curiously, there were no appreciable differences in APACHE II score between the two groups, though the influence of younger age in the APACHE II scoring system possibly compensates the differences, as has been observed in other studies.16
On analyzing the biochemical tissue inflammation and damage markers, we found the elevation of procalcitonin – as a systemic inflammatory mediator related to the severity of infectious processes and bacterial infection21 – to be an indicator of increased severity of tissue damage, hyperperfusion/hypoxia22 and augmented secondary inflammation. The reported increased incidence of thrombocytopenia23 in patients with SM is consistent with this. All the above supports the inclusion of SM within the multiorgan dysfunction syndrome associated to sepsis or septic shock.24 However, in our study other parameters that would be expected to point in this same direction failed to reach statistical significance, such as troponin and lactate. A study25 involving 93 patients suggests that natriuretic peptide elevation may be a reliable biochemical marker for predicting which patients will develop SM. In our cohort the natriuretic peptide values in the patients with SM were only slightly higher than those recorded among the patients without SM, and statistical significance was not reached. We observed no significant differences in parameters related to the level of tissue perfusion, such as lactate or central venous saturation; we are therefore unable to draw conclusions regarding the role of tissue perfusion in the physiopathology of SM.
There was no significant difference in mortality rate between the two groups, probably because of the sample size involved, though mortality did give the impression of being greater in the SM group (30.8% versus 9.1% in the group that did not develop SM). Deaths fundamentally occurred in the ICU, thereby conditioning shorter ICU stays. Although there is debate in the literature regarding the repercussions of the appearance of SM in terms of mortality,26 recent studies suggest that it is indicative of a poor prognosis.14,16,27
With regard to the echocardiographic parameters, the echocardiographic exploration upon admission revealed a statistically significant decrease in the left ventricular outflow tract velocity-time integral among the patients with SM versus those without. This is simply an echocardiographic parameter that reflects the presence of low cardiac output, and a decrease is therefore to be expected in patients that develop ventricular dysfunction in the context of sepsis. New tools such as two-dimensional speckle tracking echocardiography28 have been described for evaluating cardiac function. In this regard, a study29 involving 50 patients with sepsis and septic shock recorded alterations of the longitudinal parameters particularly in individuals with shock, even in those with preserved LVEF. This gives us a more realistic idea of cardiac dysfunction associated to sepsis and septic shock.
As expected from the definition of SM (reversible systolic dysfunction attributable to sepsis), all these parameters are corrected at patient discharge, with a return to normal levels for both LVEF and the echocardiographic recordings. Only some studies have used control echocardiography to confirm the reversibility of SM, though the publications that do so6,11 have not found the reversibility rate to be particularly high (60–70%). This is because echocardiography cannot be performed in all patients that develop SM (due either to death or to unexplained reasons).
The main limitation of our study is the limited sample size involved (57 patients). Although there are other publications with a similar number of patients, the sample is not large enough to draw firm conclusions or perform multivariate analyses. We sought to be rigorous in selecting the patients, discarding all those with any evidence of prior heart disease. This resulted in rather slow recruitment of the study sample. Another limitation is the subjectiveness of echocardiography, which can lead to some variability in interpretation, since it is an operator-dependent technique. This circumstance partly accounts for the existence of published studies with different results – hence the importance of reporting our own findings.
Myocardial dysfunction attributable to sepsis and septic shock is not infrequent, and develops in patients of increased severity due to the septic involvement of a larger number of organs, with more pronounced tissue damage. This in turn yields higher scores on the severity scales and greater procalcitonin levels in those patients that develop SM. The survivors recover heart function in the course of follow-up before hospital discharge.
Further studies involving larger patient samples are needed to draw conclusions in a field of research that has received little attention to date.
Financial supportThis study has received no financial support.
Contribution of the authorsAll the authors have made a substantial contribution to the conception and conduction of the study or interpretation of the data, as well as to the drafting, review or final approval of the manuscript.
Doctors Canabal, Narváez and Martín carried out the main field work referred to patient recruitment, the requesting of tests and measurement of variables. Doctors Giacoman and Alcalá collected most of the echocardiographic parameters. The student Alfonso Moron collaborated with data entry to the database and analysis. Doctor Sánchez-Casado performed the statistical analysis, and Dr. Magro participated in data collection.
All the authors have participated in final approval of the article.
Conflict of interestThere are no conflicts of interest.
Thanks are due to all the health professionals involved in patient care, the recording of variables and their subsequent analysis. The collaboration among different specialists and Departments proved very satisfactory.
Please cite this article as: Narváez I, Canabal A, Martín C, Sánchez M, Moron A, Alcalá J, et al. Incidencia y evolución de la miocardiopatía séptica en una cohorte de pacientes con sepsis y shock séptico. Med Intensiva. 2018;42:283–291.