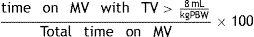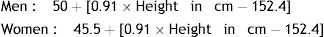To extract data from clinical information systems to automatically calculate high-resolution quality indicators to assess adherence to recommendations for low tidal volume.
DesignWe devised two indicators: the percentage of time under mechanical ventilation with excessive tidal volume (>8mL/kg predicted body weight) and the percentage of patients who received appropriate tidal volume (≤8mL/kg PBW) at least 80% of the time under mechanical ventilation. We developed an algorithm to automatically calculate these indicators from clinical information system data and analyzed associations between them and patients’ characteristics and outcomes.
SettingsThis study has been carried out in our 30-bed polyvalent intensive care unit between January 1, 2014 and November 30, 2019.
PatientsAll patients admitted to intensive care unit ventilated >72h were included.
InterventionUse data collected automatically from the clinical information systems to assess adherence to tidal volume recommendations and its outcomes.
Main variables of interestMechanical ventilation days, ICU length of stay and mortality.
ResultsOf all admitted patients, 340 met the inclusion criteria. Median percentage of time under mechanical ventilation with excessive tidal volume was 70% (23%–93%); only 22.3% of patients received appropriate tidal volume at least 80% of the time. Receiving appropriate tidal volume was associated with shorter duration of mechanical ventilation and intensive care unit stay. Patients receiving appropriate tidal volume were mostly male, younger, taller, and less severely ill. Adjusted intensive care unit mortality did not differ according to percentage of time with excessive tidal volume or to receiving appropriate tidal volume at least 80% of the time.
ConclusionsAutomatic calculation of process-of-care indicators from clinical information systems high-resolution data can provide an accurate and continuous measure of adherence to recommendations. Adherence to tidal volume recommendations was associated with shorter duration of mechanical ventilation and intensive care unit stay.
Extraer los datos del Sistema de Información Clínica para calcular automáticamente indicadores de calidad de alta resolución para evaluar la adherencia a las recomendaciones sobre el volumen tidal.
DiseñoIdeamos 2 indicadores: el porcentaje de tiempo en ventilación mecánica con volumen tidal excesivo (>8mL/kg peso ideal) y el porcentaje de pacientes con volumen tidal apropiado (≤8mL/kg peso ideal) al menos el 80% del tiempo en ventilación mecánica. Desarrollamos un algoritmo para calcular automáticamente dichos indicadores con los datos del Sistema de Información Clínica y analizamos su asociación con las características de los pacientes y su evolución.
AmbienteEl estudio se llevó a cabo en una unidad de cuidados intensivos polivalente de 30 camas desde el 1 enero 2014 hasta el 20 noviembre 2019.
PacientesSe incluyeron en el estudio todos los pacientes ingresados en la unidad de cuidados intensivos conectados a ventilación mecánica>72h.
IntervenciónUsar los datos recogidos automáticamente desde el Sistema de Información Clínica para evaluar la adherencia a las recomendaciones del volumen tidal y sus resultados.
Principales variables de interésDías de ventilación mecánica, días de estancia en la unidad de cuidados intensivos y mortalidad.
ResultadosDe todos los pacientes ingresados, 340 cumplieron los criterios de inclusión. El tiempo medio de ventilación mecánica con volumen tidal excesivo fue 70% (23-93%); solo el 22,3% de los pacientes recibió un volumen tidal apropiado al menos el 80% del tiempo. Recibir un volumen tidal apropiado se asoció con menos días de ventilación mecánica y de estancia en la unidad de cuidados intensivos. Los pacientes que recibieron un volumen tidal apropiado fueron más frecuentemente hombres, más jóvenes, más altos y menos graves. No hubo diferencias significativas en la mortalidad ajustada en relación con el porcentaje de tiempo de volumen tidal excesivo o recibir un volumen tidal apropiado al menos el 80% del tiempo.
ConclusionesEl cálculo automático de los indicadores de calidad desde el Sistema de Información Clínica puede proporcionarnos una medida precisa y continua de la adherencia a las recomendaciones. La adherencia a las recomendaciones sobre el volumen tidal se asocia con menos días de ventilación mecánica y de estancia en la unidad de cuidados intensivos.
A growing number of healthcare performance measures are being publicly reported. Patients, providers, payers and policymakers deserve valid, reliable and transparent quality measures.1 Indicators are the best tools for measuring quality, but collecting the information needed to calculate them is time consuming and complex.2 Information technology can provide to critical care with new tools to improve management, decision making and effectiveness of care.3
Indicators should be measurable, reliable, valid and reproducible.4 Using indicators based on data extracted from the clinical information systems (CIS) can help ensure homogeneous definitions and reduce the time professionals need to invest in collecting data. Recently, our group showed that it is feasible to automatically generate the minimum dataset and intensive care unit (ICU) quality indicators with a data management tool we developed using business discovery techniques on an associative data model created from variables stored in the CIS.5
Ventilator management is an essential part of critical care. However, mechanical ventilation (MV) probably can aggravate acute lung injury as ventilator-induced lung injury (VILI), especially in patients with acute respiratory distress syndrome (ARDS).6 As high tidal volume (TV) has been demonstrated to be prejudicial and produced volutrauma,7 the standard of care in these patients is protective mechanical ventilation with low TV (<8mL/kg predicted body weight (PBW)),8 reducing mortality by 22%.9 Moreover, some studies conclude that protective mechanical ventilation is not only beneficial for patients with ARDS, but also improve outcomes in patients with healthy lungs.10,11 A recent large epidemiologic study, LUNG SAFE, concluded that ARDS is underdiagnosed and often goes unrecognized until after significant delays.12 Growing evidence supports the use of low TV as early as possible in patients with acute respiratory failure regardless of whether ARDS has been diagnosed.10 However, different studies show that patients with ARDS do not consistently receive low TV despite over 15 years’ effort to ensure its adoption into clinical practice.13–15
Our approach consists in taking profit of all the continuous measurements automatically collected by the CIS to create high-resolution quality indicators (HR-QI) to assess TV, improving snapshot-based assessments and saving time to healthcare professionals.
Our main objective was to define, implement and evaluate two HR-QI to assess adherence to clinical practice guidelines for protective mechanical ventilation in our ICU using data automatically collected in our CIS database. Our secondary objective was to analyze patients’ characteristics according to those HR-QI and its impact on outcomes.
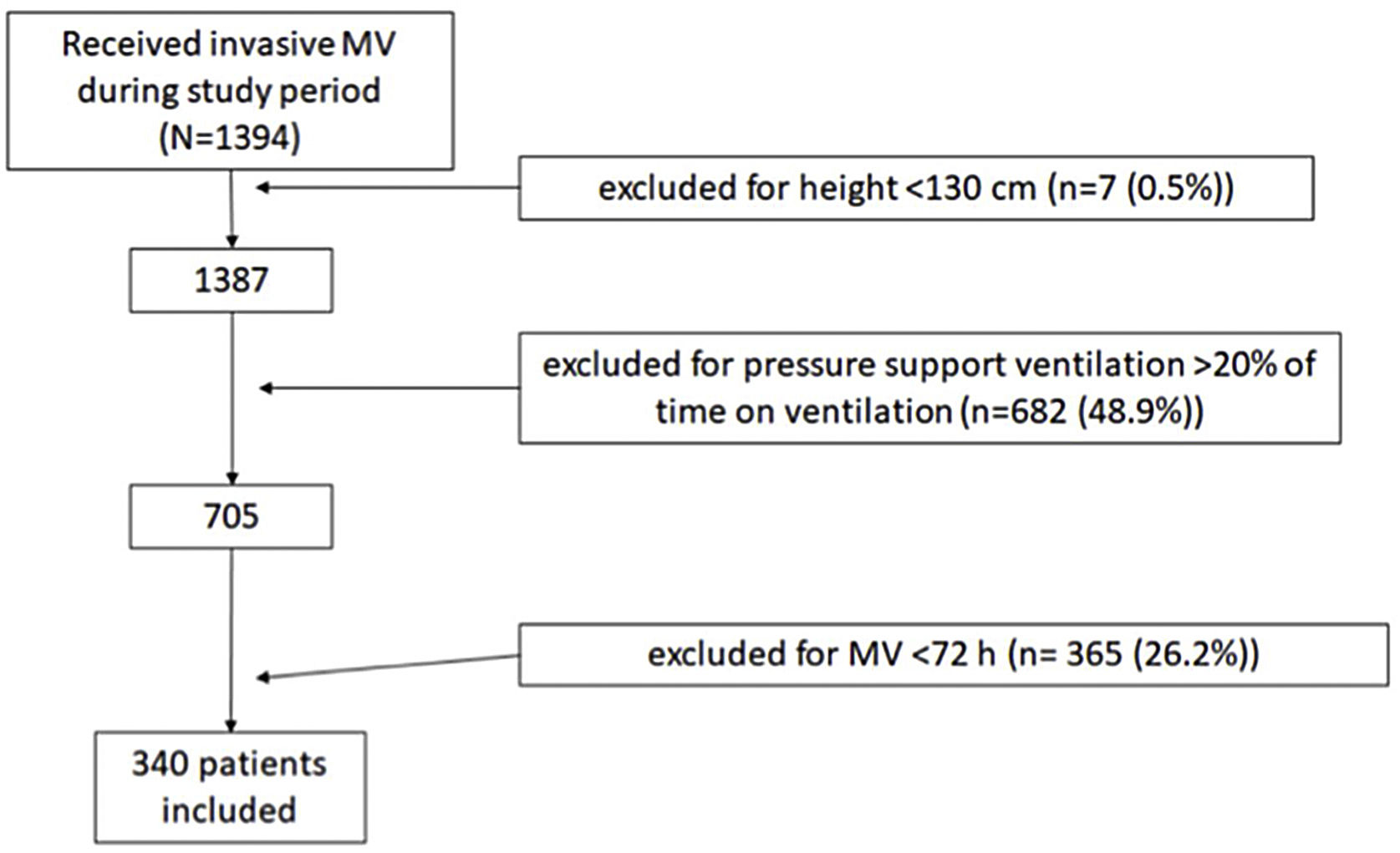
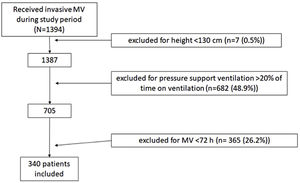
Patients and methodsDesignWe included all non-coronary patients who received invasive MV in our 30-bed polyvalent ICU Hopital Universitario Joan XXIII (Tarragona) between January 1, 2014 and November 30, 2019. We did not include coronary patients because they are attended by other specialists. We excluded patients whose height and/or weight was not recorded and those <130cm tall, for whom the PBW equation may not apply.16 We also excluded patients who received MV<72h because they were not at risk for prolonged exposure to high TV and those ventilated with pressure support more than 20% of the time, because we were interested in TV delivery directly set by the physicians.
All patients or their legal representatives provided written informed consent. Our center's research ethics committee approved the study protocol (CEIm Institut d’Investigació Sanitària Virgili - Reference IRB#41/2016).
Patient data captureSince 2013, our ICU has been using a commercial CIS (Centricity Critical Care® from General Electric) to enter orders, document clinical acts, record medication administration and collect data. Moreover, mechanical ventilators are connected to the CIS, and all respiratory parameters are recorded every 2min. All inputs are stored in a data storage repository. All data used in this study have been extracted from the CIS database by means of ETL (extraction, transform and load) processes implemented with Python 3.
Clinical variablesWe extracted the following variables: age, sex, admission source, reason for admission (classification according to variables of the Minimum Data Set of Intensive Care Unit CMBD-UCI of the Sociedad Española de Medicina Intensiva y Unidades Coronarias (SEMICYUC) criteria5) (Supplementary Table 1), patient type (medical or surgical), admission type (urgent or scheduled), height, weight, APACHE II and the worst values of clinical variables as mean arterial pressure, body temperature, heart rate, pulse oximetry (SpO2), pH, PaCO2, serum lactate, serum bicarbonate, Richmond Agitation Sedation Scale (RASS), Sequential Organ Failure Assessment (SOFA) score, administration of vasopressor drugs, continuous renal replacement therapy (CRRT) and administration of analgesics and sedatives in the first 48h of MV.
Ventilatory variablesWe analyzed the set and/or observed values of the following ventilatory variables: TV, positive end-expiratory pressure (PEEP), peak pressure (Ppeak), plateau pressure (Pplat), respiratory rate (RR) and fraction of inspired oxygen (FiO2). All ventilation variables were extracted as median values for each hour during the first 48h after starting MV (Table 1). For calculate HR-QI we have recorded TV during the entire time in MV.
Characteristics of patients included in the study (n=340).
| Variables | Values |
|---|---|
| Demographics (at admission) | |
| Age (years), median (p25–75) | 58.4 (48.8–71.0) |
| Male sex, n (%) | 234 (68.8) |
| Weight (kg), median (p25–75) | 75.0 (70–85.0) |
| Height (cm), median (p25–75) | 170 (165–175) |
| Admission type | |
| Medical admission, n (%) | 220 (64.7) |
| Emergency surgical admission, n (%) | 112 (32.9) |
| Elective surgical admission, n (%) | 8 (2.4) |
| Reason for admission | |
| Respiratory failure, n (%) | 82 (24.1) |
| Sepsis, n (%) | 51 (15) |
| Others, n (%) | 207 (60.9) |
| Severity scores (at 24h of admission) | |
| APACHEaII score(points), median (p25–75) | 24 (19–30) |
| SOFAbscore (points), median (p25–75) | 8 (6–10) |
| Respiratory and mechanical ventilation (first 48h) | |
| SpO2c(%) | 96 (94–98) |
| SpO2/FiO2, median (p25–75) | 240 (160–312.9) |
| FiO2d(%) | 35 (30–45) |
| % controlled MV modes | 93 (86–99) |
| Set MVeflow (L/min), median (p25–75) | 60 (50–60) |
| Positive end-expiratory pressure (cmH2O), median (p25–75) | 5 (5–8) |
| Peak pressure (cmH2O), median (p25–75) | 24 (22–28) |
| Plateau pressure (cmH2O), median (p25–75) | 20 (17–25) |
| Observed respiratory rate (breaths/min), median (p25–75) | 17 (16–19) |
| Set respiratory rate (breaths/min), median (p25–75) | 18 (16–20) |
| Set tidal volume (mL), median (p25–75) | 520 (480–552) |
| Observed tidal volume (mL), median (p25–75) | 520 (480–560) |
| Set tidal volume (mL/predicted body weight), median (p25–75) | 6.8 (6.0–7.6) |
| Observed tidal volume (mL/predicted body weight), median (p25–75) | 6.8 (5.9–7.5) |
| Clinical and laboratory variables (first 48h) | |
| Heart rate (beats per minute), median (p25–75) | 102 (87–118) |
| Mean arterial pressure (mmHg), median (p25–75) | 68 (63–72) |
| Serum lactate (mmol/L), median (p25–75) | 2.1 (1.5–3.0) |
| Body temperature (°C), median (p25–75) | 37 (36.5–37.5) |
| Richmond Agitation-Sedation Scale (points), median (p25–75) | −3 (−3 to −2) |
| Treatments and outcome (first 48h) | |
| Vasoactive drugs, n (%) | 243 (71.5) |
| Sedative drugs, n (%) | 318 (93.5) |
| Neuromuscular blocking agents, n (%) | 47 (13.83) |
| Continuous renal replacement, n (%) | 42 (12.4) |
| Died in ICU, n (%) | 151 (44.4) |
| Died in hospital, n (%) | 156 (45.9) |
Data are shown as median (interquartile range) or number (percentage) of patients, as appropriate.
Because PaO2 was not recorded in all patients, instead of PaO2/FiO2 we used pulse oximetry (SpO2)/FiO2 [S/F] ratio, which correlates acceptably with PaO2/FiO2.17
Primary endpointsWe designed two HR-QI to perform a high-resolution assessment of the adherence to low TV recommendations defined as follows:
- 1)
Percentage of time on MV with excessive TV (%tTVot), defined as the time under MV in which the TV is above the recommended values [> 8mL/kg PBW] and calculated according to the formula:
- 2)
Percentage of patients who received appropriate TV (%pTVa), calculated according to the formula:
PBW was calculated according to the formulas:
Secondary endpointsDuration of invasive MV, defined as the number of days between the date of intubation and the date of MV disconnection (picked by a nurse the first day of MV disconnection into the CIS) or death; ICU length of stay (LOS), defined as the number of days between the date of admission to the ICU and the date of discharge from the ICU; hospital LOS, defined as the number of days between the date of admission to the hospital and the date of discharge from the hospital; and ICU and hospital mortality.
Statistical analysisCategorical variables are expressed as counts (percentage) and continuous variables as medians (interquartile range). To compare patient demographic and clinical characteristics between two groups, we used the chi-square test or Fisher's exact test for categorical variables, as appropriate, and Student's t-test or the Mann–Whitney U test for continuous variables. For univariate comparisons with more than two groups, we used the chi-square test for categorical variables and the Kruskal–Wallis test for continuous variables. Multivariate comparisons were performed using binary logistic regression and models were evaluated using its accuracy and area under the receiver operating characteristic curve (AUC). Logistic regression coefficients were converted to odds ratios to easy interpretability of covariates influence in each group.
To avoid spurious significance between variables related to the large volume of data analyzed, we set significance at p<0.005.18
To characterize patients’ profile according to our first HR-QI %tTVot, patients were categorized into quartiles. To investigate the association between baseline variables (at ICU admission and first 48h of invasive MV) and %tTVot, we first performed univariate analysis. Afterwards, we used binary logistic regression to compare the fourth (highest) quartile against the combined group of patients in the first, second and third quartiles in a multivariate model including only demographics and severity covariates that were significant in the univariate analysis.
To characterize patients’ profile according to our second HR-QI %pTVa, patients were divided in two groups, those who accomplished the HR-QI and those who do not. To investigate the association between baseline variables and the two groups, we first performed univariate analysis. Afterwards, we used binary logistic regression to compare them in a multivariate model including only demographics and severity covariates that were significant in the univariate analysis.
To evaluate the association between inappropriate ventilation (according to our two HR-QI) and mortality, we used univariate and binary logistic regression analysis following the same procedure but including both HR-QI in the final analysis. Analyses were done with Python (Python Software Foundation – Python.org) and R (CRAN-R project) software.
ResultsPopulation characteristicsWe analyzed data from 340 patients (Fig. 1) whose median age was 58.4 (48.8–71.0) years; 235 (69%) were men with median APACHE II score of 24 (19–30) points and median SOFA score of 8 (6–10) points (Table 1).
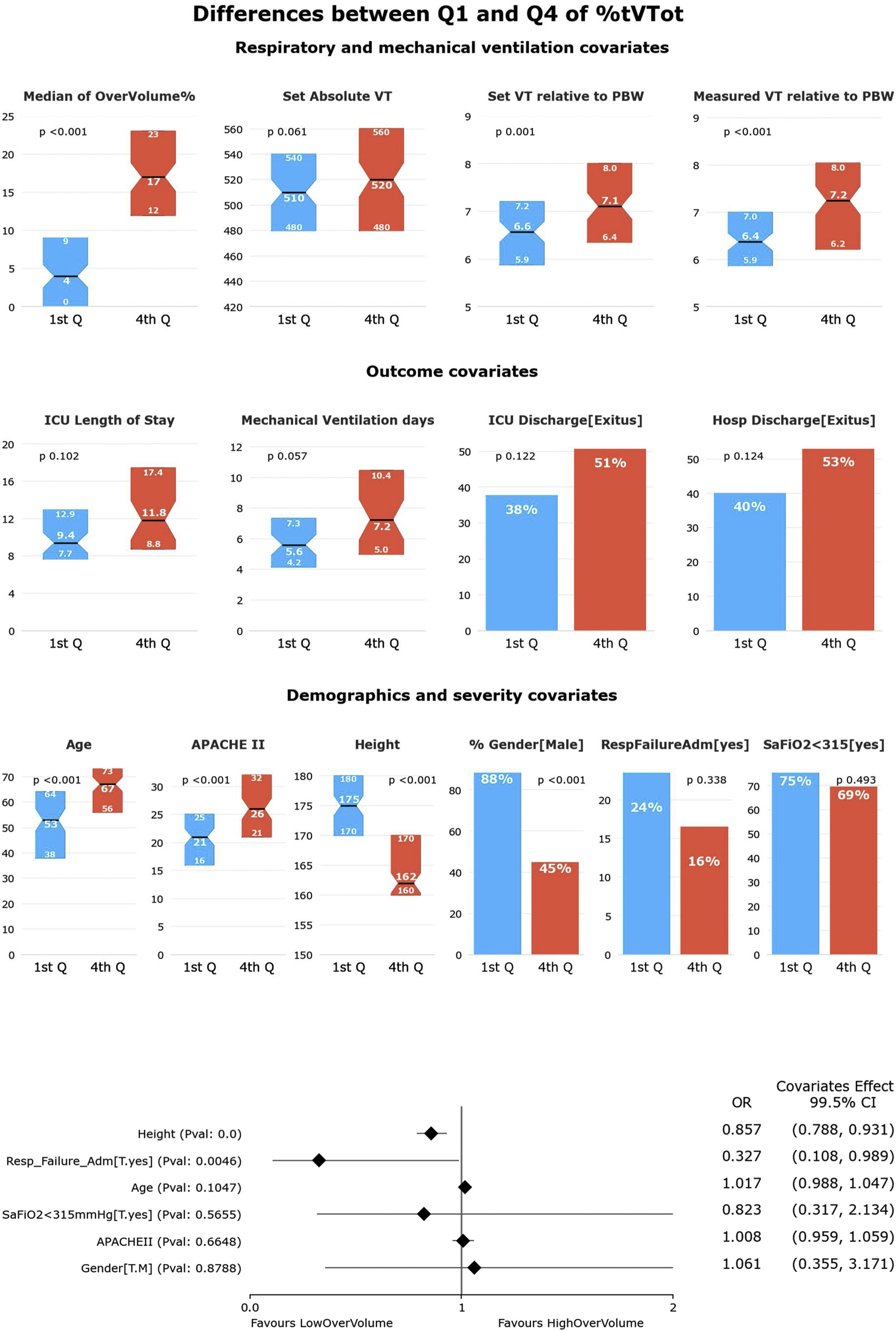
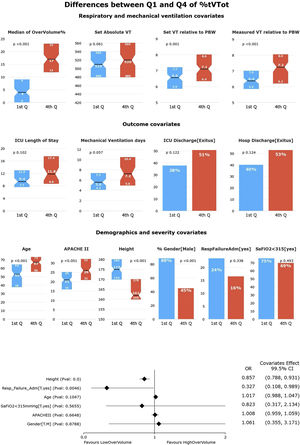
HR-QI (%tTVot)The results of our first HR-QI showed a median %tTVot of 70% (23%–93%), with a median excessive volume of 8% (3%–15%) over the amount required according to patient's PBW. Regarding to patient's characterization according to our first HR-QI, patients in the first quartile included a greater proportion of men and were younger, taller, and less severely ill (p<0.001) in comparison with the rest of quartiles. Comparing the first against the fourth quartile, the median excessive volume over the one required according to their PBW was lower [4% vs. 17%, respectively, p<0.001]. It is important to highlight that no differences were found in the set absolute TV amount (mL) between the extreme quartiles (p=0.06), suggesting that the relative amount to PBW (and therefore the height) is what makes the big difference (p=0.001 and p<0.001 for set and measured TV relative to PWB respectively). No other differences were observed in respiratory or MV variables. Even if ICU LOS, duration of MV and ICU and hospital mortality were higher in the fourth quartile than in the first, those differences were not significant (Fig. 2 and Supplementary Table 2).
Univariate and multivariate differences for HR-Q1 (%tTVot).
In the univariate analysis, patients in Q4 (highest %tTVot) have higher set and measured TV relative to PBW, but not in absolute TV. There is no significant difference between Q1 and Q4 in outcome covariates. There is no difference in the distribution of patients with SaFiO2<315 or admitted for respiratory failure in the quartiles. Patients in Q1 were mostly male, younger, taller, and less severely ill (significance at p<0.005).
In the multivariate analysis, height was the most associated variable with %tTVot. (significance at p<0.005).
In the multivariate analysis, only height was independently associated with %tTVot (Fig. 2). We obtained an accuracy of 77.94% and an AUC curve of 0.81 (0.74–0.86) (Supplementary Figure 1).
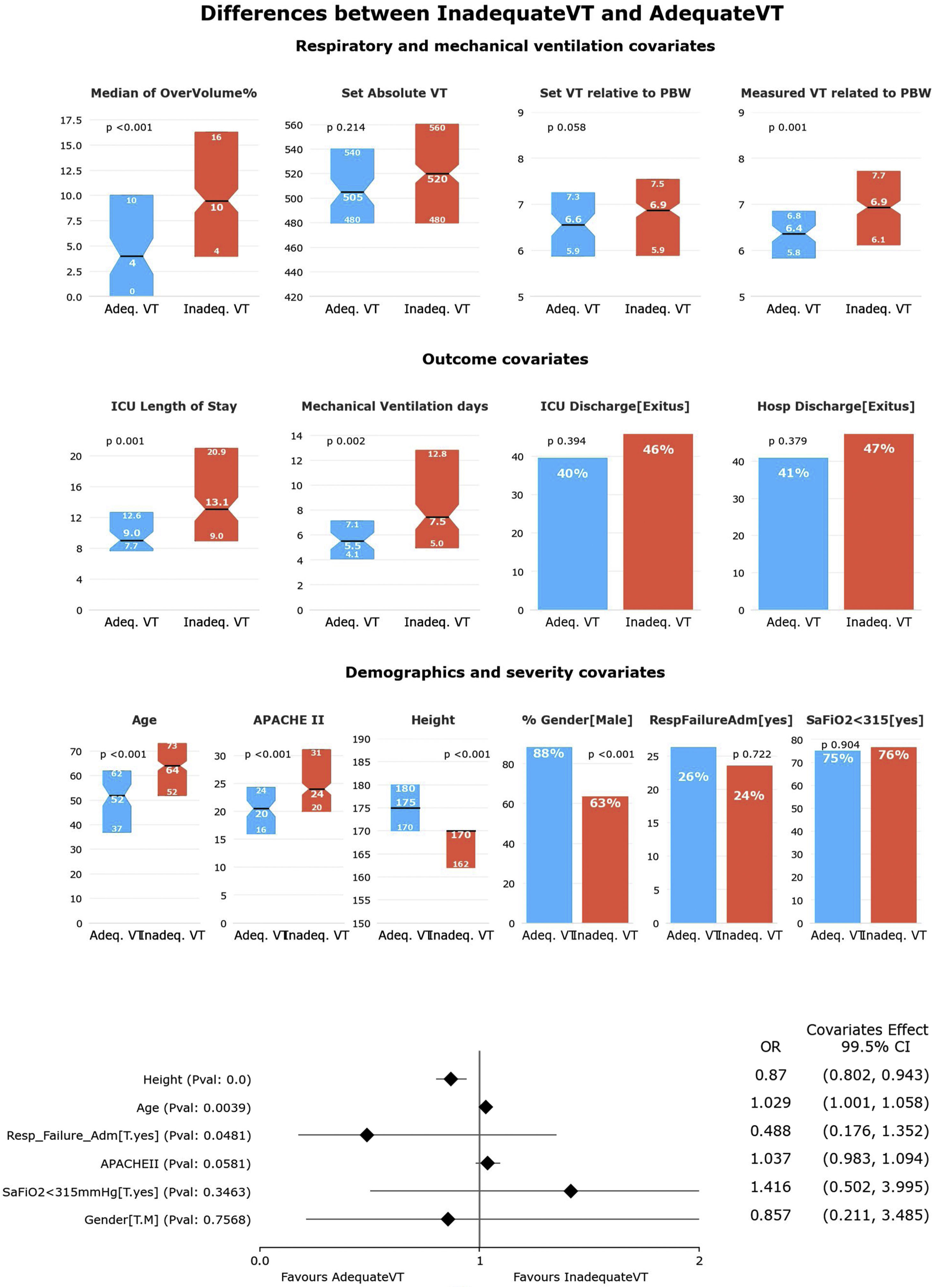
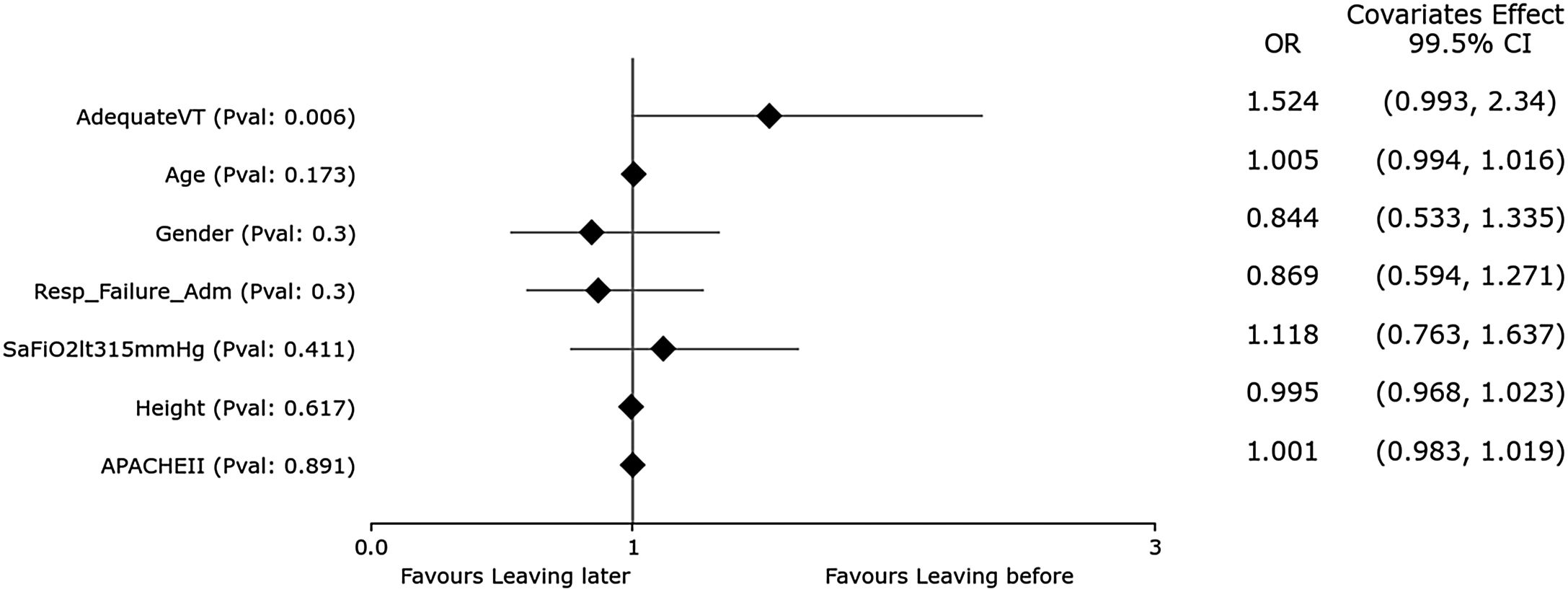
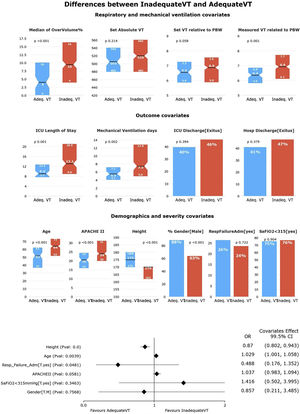
Second HR-QI (%pTVa)Of the 340 patients analyzed, only 76 (22.3%) received appropriate TV according to the definitions of our second HR-QI. Patients receiving appropriate TV were younger and taller, with a greater predominance of men and lower severity of illness. As expected, %tTVot and excessive volume were higher in the group receiving inappropriate TV.
Although set TV did not differ between groups, measured TV adjusted for PBW was higher in the group receiving inappropriate TV (Fig. 3). Duration of MV and ICU LOS were significatively shorter in the group receiving appropriate TV (p<0.05) (Figs. 4 and 5). No differences in ICU mortality were observed between the groups (Fig. 3 and Supplementary Table 3).
Univariate and multivariate differences for HR-Q2 (%pTVa).
In the univariate analysis, patients receiving appropriate TV (TVa) have shorter ICU length of stay and less mechanical ventilation days. There is no difference in the distribution of patients with SaFiO2<315 or admitted for respiratory failure in the quartiles. Patients receiving TVa were mostly male, younger, taller, and less severely ill (significance at p<0.005).
In the multivariate analysis, only height was independently associated with having an adequate TV (significance at p<0.005).
In the multivariate analysis, only height was associated with having an adequate TV (Fig. 3). We obtained an accuracy of 80.59%and an AUC curve of 0.82 (0.76–0.87) (Supplementary Figure 2).
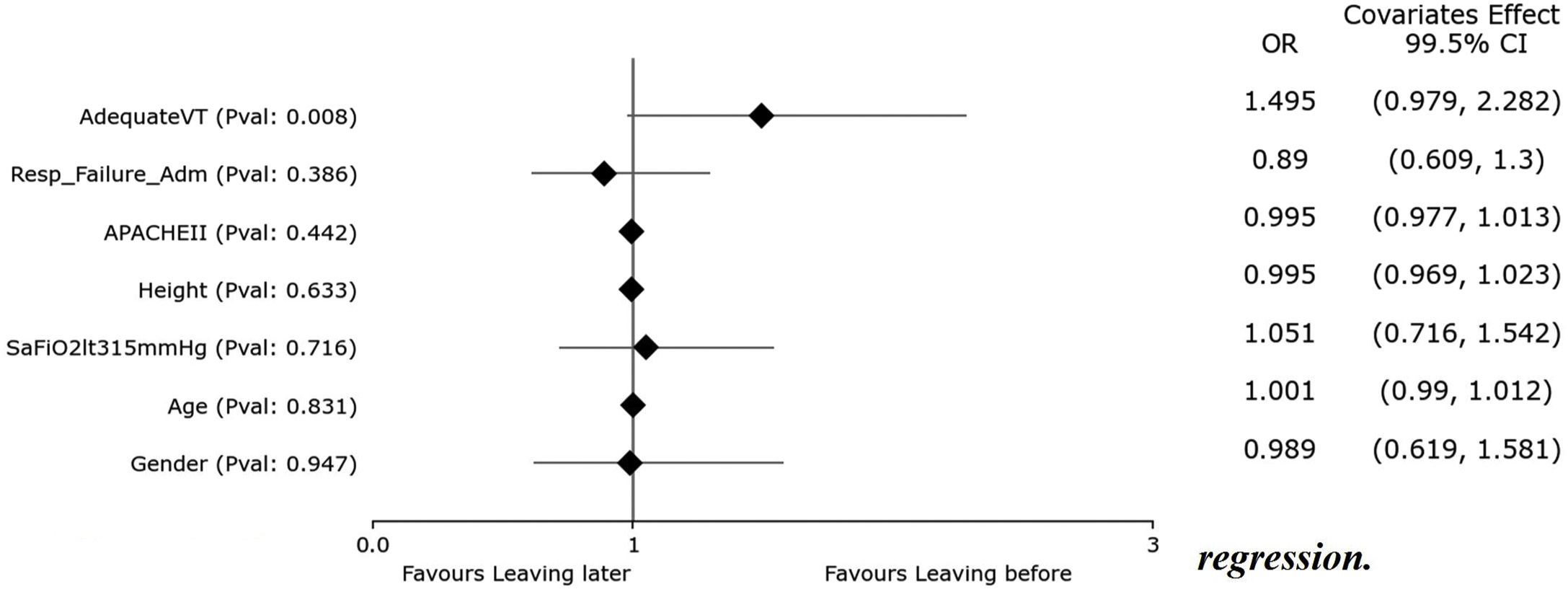
Variables independently associated with ICU mortalityThe crude mortality rate was 44.4% in the ICU and 45.9% in the hospital (Table 1). Patients who died were older (73.5 vs. 62.9), with higher APACHE II score (25 vs. 22), higher lactate concentration (2.3 vs 1.9) and lower SpO2 level (95 vs. 96) (Supplementary Table 4).
We included all these 4 significant variables resulted from the univariate analysis together with our HR-QI target variables: %tTVot and TVa. We found that only age was independently associated with mortality (Supplementary Figure 3). We obtained an accuracy of 67.94% and an AUC curve of 0.74 (0.68–0.8) (Supplementary Figure 4).
DiscussionTechnological advances since the first CIS and patient data management systems, which were introduced in the late 1980s,19 have enabled the integration of a wide range of bedside devices and automatic data collection.20 To date, systematic reviews on using CIS have concentrated primarily on their organizational impact (e.g. charting, documenting, patient care, etc.) rather than their impact on clinical outcomes and quality assessment.21,22
Our results support the view that the secondary use of data from the CIS can be very useful to assess adherence to clinical practice guidelines23,24 and create more accurate quality indicators, helping us to improve the process of care in our ICU. We adapted current definitions of indicators to allow them to be automatically calculated with data from our CIS.25
We have been able to perform a high-resolution evaluation of the adherence to recommendations for low TV in MV using data automatically and continuously collected (one measurement each 2min) into the CIS. Since MV is dynamic and changeable 24h a day, data extracted in other studies where respiratory variables have been measured once or twice a day,10,12,13 provide an incomplete picture of compliance with recommendations for protective mechanical ventilation. Our group have also carried out different studies analyzing the efficiency of random safety analysis on structure, process and outcome indicators, including protective mechanical ventilation, without detecting such a great lack of adherence as the CIS.26,27 Probably both methodologies are complementary.
Despite several studies reported benefits of low TV and it is recommended in clinical practice guidelines, there is poor adherence to them.13–15 We found that, on average, our patients received TV above the recommended cutoff (8mL/kg PBW) 70% of time they were under MV. These results are in line with those reported in other studies.28 Among various possible explanations for these findings, one that stands out is the use of actual body weight instead of PBW to set TV. Using PBW seeks to minimize volutrauma by better estimating the patient's lung capacity; lung capacity and respiratory system compliance relate more closely to height than to weight.16 We suspect that the main reason for not following guidelines on lower TV in our series was inaccurate calculation of PBW. In fact, we have observed that shorter patients and women (generally shorter than men) were more common ventilated with higher TV, as found in other studies.12,14,29,30 In a secondary analysis of data from the LUNG SAFE study, McNicholas et al.31 recently demonstrated important sex differences in the management and outcomes of patients with ARDS; lower TV was applied in only half the female patients, and shorter women more likely to receive higher TV than shorter men. Moreover, mortality rates were significantly higher in women.
We observed that the duration of MV and ICU LOS were higher in patients with higher percentage of time on MV with TV above the target level and in those patients who received TV above the target level for more than 80% of the time, correlating with previous literature.28 We found no association between the proportion of time with high TV and mortality. These findings are likely due to other factors that can impact mortality; for example, Serpa Neto et al. demonstrated that, even at low TV and low driving pressure, high mechanical power is associated with worse outcomes.32 Although our study have been focused on TV, CIS can also calculate driving pressure and mechanical power values in a continuous way, that would be studied in the future.
It is widely recognized that protective mechanical ventilation with lower TV is associated with better clinical outcomes in patients with ARDS.9 Although most studies have shown that high TV is associated with increased complications also in patients with healthy lungs,10,11 evidence supporting protective mechanical ventilation in patients without ARDS is inconclusive.33 However, there are good reasons to strongly consider using low TV in all patients,13 even at the initiation of MV.28 Lung damage can occur within hours of initiating MV with inappropriate settings, ARDS is often unrecognized until after a delayed onset inflammatory process and critically ill patients are at risk of other causes of lung injury. Therefore, in addition to a therapeutic modality, low TV can be useful as a preventive measure, especially in patients with conditions involving increased risk of lung injury, such as sepsis, trauma, or high-risk surgeries.34
Quality indicators proposed for respiratory care and MV did not include protective mechanical ventilation.35,36 However, considering findings of higher mortality in ARDS patients ventilated with high TV in the last decade,9,12 more recent quality-control guidelines from various countries include indicators related to protective mechanical ventilation,37,38 although some refer only to indicators of structure, for example the availability of a written protocol or routine for a lung-protective ventilatory strategy.39 One reason why indicators that could provide better information about MV processes are not implemented is that accurate information to measure them is unavailable or difficult to obtain. Our study shows that this problem can be overcome.
Our study has important limitations that must be pointed out. First, our analysis included only patients who received volume-controlled MV. We did not analyze patients receiving pressure-controlled or pressure-support ventilation, because in this context TV is influenced by the applied airway pressures as well as the compliance of the respiratory system,40 increasing the margin of error in determining actual TV set or in analyzing its impact on outcomes. Second, we did not consider the characteristics of MV in the emergency room or operating room prior to ICU admission, which may affect outcomes41; however, including only patients undergoing >72h MV in the ICU probably reduced the impact of ventilation outside the ICU on outcomes drastically. Furthermore, because we were unable to calculate PaO2/FiO2 in all patients, we used SaO2/FiO2 instead; SaO2/FiO2 is accessible and reliable, can be obtained continuously, and correlates acceptably with PaO2/FiO2.17 Nevertheless, our study has the strength of using continuous data from a very homogeneous population of ventilated patients. Data-based decision making depends on the quality of the data and we have taken steps to guarantee their quality. An earlier study demonstrated that none of the variables collected by our data management tool differed significantly from those collected manually by trained staff.5
ConclusionThere is low adherence to clinical practice guidelines related to protective mechanical ventilation. The amount TV over target time and the amount of excessive TV worsen patients’ outcomes. Men, taller, younger, and less severely ill are better ventilated according to clinical practice guidelines, which suggest that PBW needs to be calculated more carefully in our unit. Data extracted from the CIS can provide invaluable information about deviations from recommended clinical practice. Automatically generating HR-QI allowed us to identify actions to improve the quality of care in our ICU, sparing professionals the tedious, time-consuming tasks of collecting data and calculating indicators.
Clinical relevance statementOur study demonstrates how the data stored into the Electronic Health Records through the Clinical Information Systems can be exploited to build high-resolution quality indicators (HR-QI) of care without any extra efforts for the healthcare professionals. We evaluated two HR-QI to assess tidal volume and its impact on outcomes.
Ethics approvalThe study was approved by the Ethics and Clinical Research Committee (CEIm Institut d’Investigació Sanitària Virgili – Reference IRB#41/2016).
Code availabilityThe code for the current study is available from the corresponding author on reasonable request.
FundingThis study was supported by grants from the Fondo de Investigación Sanitaria (Institute of Health Carlos III from Spain, FIS grants, project PI20/01674) and Plá estratègic de reserca i innovació en salut (PERIS SLT017/20/000030).
Authors’ contributions- -
Conceptualization: María Bodí, Alejandro Rodríguez, Sara Manrique.
- -
Data curation; Formal analysis; Software: Josep Gomez, Manuel Ruiz-Botella.
- -
Funding acquisition: María Bodí.
- -
Methodology: María Bodí, Federico Gordo.
- -
Supervision: María Bodí, Josep Gomez.
- -
Validation: Josep Gomez.
- -
Writing – original draft: Sara Manrique, Alejandro Rodríguez, Manuel Ruiz-Botella, Federico Gordo.
- -
Writing – review and editing: Alejandro Rodríguez, María Bodí, Josep Gomez.
None.
Advanced Analysis of Critical Data (AACD) research group:
Physicians: María Bodi, Alejandro Rodríguez, Gerard Moreno, Christian Villavicencio, Mari Carmen Gilavert, Sara Rosich, Ángel Pobo, Mónica Magret, Gonzalo Sirgo, Laura Claverías, Vanessa Blázquez, Federico Esteban, Iulen Leache, Paula Perello, Iban Oliva, Manuel Samper, Oriol Plans, Marc Cartanyá, Sandra Canelles, Raquel Carbonell.
UCI Data-Analitics: Josep Gómez, Manuel Ruiz-Botella, Jordi Albiol, Eduard Mayol.
John Giba edited the manuscript.