Aneurismal subarachnoid hemorrhage (aSAH) is the most common cause of non-traumatic extravasated bleeding into the subarachnoid space and the main cause of sudden death from stroke.1 Due to its high rate of mortality and morbidity, researchers have focused their efforts on improving clinical management, imaging techniques and identifying biomarkers that could early predict cerebral vasospam, delayed cerebral ischemia and aSAH patients’ outcome.2 One of these biomarkers is gelsolin, a protein with a protective function: promotes the clearance of actin filaments released during tissue injury.3 Its depletion has been related to poor outcome in various critical care pathologies.4
The aim of this preliminary study was to analyze gelsolin serum levels in patients with aSAH, describe its kinetic within 48h post-bleeding and assess its role as a possible outcome predictor.
For this purpose, we included patients admitted to the NeuroCritical Care Unit (NCCU) at Virgen del Rocío University Hospital in Seville, Spain, with the diagnosis of aSAH during a 5-month long period. The protocol, carried out in accordance with the Declaration of Helsinki, was approved by our hospital Institution Review Board. Written informed consent was obtained from patients family members. aSAH were eligible based on the following inclusion criteria: aged 18 or over, clinical history of SAH with evidence of bleeding on CT scan, NCCU admission within 24h of onset, absence of tumor, trauma, previous episodes of SAH or any other neurological disease that could modify results. Clinical and demographic variables, collected prospectively, included: gender, age, Glasgow Coma Scale (GCS), Hunt and Hess (HH) classification, World Federation of Neurological Surgeons (WFNS) scale, Modified Fisher grade and 6-month mortality.
During the recruitment period, 15 aSAH patients fulfilled the inclusion criteria. Venous blood samples were collected on admission and 48h after the bleeding. Additionally, a group of 10 healthy subjects were volunteers to extract a blood sample.
Serum gelsolin was measured using a double-antibody sandwich commercialized by Cloud-Clone Corp. (CCC, USA). Shapiro–Wilk normality test stated that gelsolin data followed a normal distribution. Hence, values were presented as means and standard deviation (SD). Comparison of means of quantitative variables between groups were made applying Student's t test. Correlation of gelsolin levels with other variables was assessed by Pearson's correlation coefficient. Statistical significance was defined as p<0.05. Statistical analyses were performed using SPSS V20 software (IBM Inc., Chicago, IL, USA).
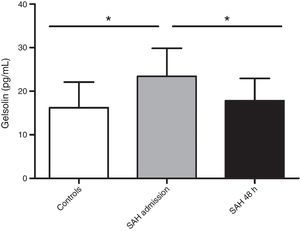
Clinical characteristics are recorded in Table 1. Death in the 6 non-survivors patients occurred at mean day 44 (range 21.75–68.50). All deaths were direct effect of the SAH. No patient suffered hydrocephalus during the studied period. aSAH patients’ gelsolin serum levels at admission were significantly increased (23.43±6.42pg/mL) compared with healthy control subjects (16.20±5.89pg/mL) (p=0.009). In reference to the 48-hour sample, gelsolin levels significantly decreased with respect to admission sample (17.80±5.14pg/mL) (p=0.031) (Fig. 1). When we grouped patients according to 6-month mortality, we found a significant difference between controls and admission samples from deceased (23.81±5.24pg/mL) (p=0.021) and between controls and survivors (23.17±7.4pg/mL) (p=0.036). The present data showed no difference on gelsolin values between survivors and non-survivors.
Characteristics, demographics and clinical data of 15 aneurysmal subarachnoid hemorrhage patients.
| Clinical variable | aSAH patients n=15 | Survivors n=9 | Non-survivors n=6 |
|---|---|---|---|
| Gender, male, n (%) | 7 (46.7) | 3 (33.3) | 4 (66.7) |
| Age, median (IQR) | 56 (50–63) | 50 (44.00–58.50) | 63.50 (56.25–65.25) |
| GCS score, n (%) | |||
| 3–8 | 8 (53.3) | 5 (55.6) | 3 (50.0) |
| 9–14 | 2 (13.4) | 1 (11.1) | 1 (16.7) |
| 15 | 5 (33.3) | 3 (33.3) | 2 (33.3) |
| HH, n (%) | |||
| II | 4 (26.7) | 3 (33.3) | 1 (16.7) |
| III | 2 (13.3) | 0 (0.0) | 2 (33.3) |
| IV | 7 (46.7) | 5 (55.6) | 2 (33.3) |
| V | 2 (13.3) | 1 (11.1) | 1 (16.7) |
| WFNS, n (%) | |||
| I | 2 (13.3) | 2 (22.2) | 0 (0.0) |
| II | 3 (20.0) | 1 (11.1) | 2 (33.3) |
| III | 1 (6.7) | 0 (0.0) | 1 (16.7) |
| IV | 6 (40.0) | 5 (55.6) | 1 (16.7) |
| V | 3 (20.0) | 1 (11.1) | 2 (33.3) |
| Modified Fisher, n (%) | |||
| III | 6 (40) | 5 (55.6) | 1 (16.7) |
| IV | 9 (60) | 4 (44.4) | 5 (83.3) |
| Rebleeding, n (%) | 5 (33.3) | 2 (22.2) | 3 (50.0) |
| Neurosurgery within 48h, n (%) | 3 (20.0) | 3 (33.3) | 0 (0.0) |
Correlation analysis demonstrated the existence of significant correlation between gelsolin serum levels at 48h of the bleeding and two severity scales: negative correlation with the GCS score (r=−0.597, p=0.019) and positive correlation with HH scale (r=0.554, p=0.032). Gelsolin values did not vary according to the development of rebleeding or the performance of neurosurgery within 48h of the initial bleeding.
To the authors’ knowledge, just few studies have analyzed the role of gelsolin in aSAH. Kulakowska et al. measured gelsolin with two assays (immunoblotting and fluorometric actin polymerization) in cerebrospinal fluid (CSF) and serum from patients with different neurological disorders, and found that patients recovering from SAH had lower gelsolin levels compared to the other neurological pathologies.5 Few years later, Chou et al. analyzed gelsolin in 42 SAH patients, reporting a decrease in blood gelsolin levels in SAH patients compared with controls.6 Later, Pan et al. performed a study with the largest SAH population to date to analyze gelsoline role.7 Similarly to previous findings, authors concluded that blood gelsolin levels on admission from SAH patients were significantly lower compared to healthy controls. Moreover, they found a significant difference in gelsolin levels depending on 6-month GOS (survivors vs. deceased; good vs. poor outcome). Their correlation analysis stated a negative correlation between gelsolin and WFNS and Fisher scores. These results do not agree with the ones obtained in the present study. Nevertheless we should highlight the differences on the assay employed to measure gelsolin. Pan et al. measured gelsolin in plasma, using an enzyme-linked immunosorbent assay commercialized by CoTimes (Beijing, China). While they referred a mean value for gelsolin in healthy subjects of 126.4±35.4mg/L, our results state 16.20±5.89pg/mL for controls. This difference rounds 5 orders of magnitude, hence it should be necessary to analyze concordance and correlation between both gelsolin assays before comparing these studies results.8 Additionally, we measured gelsolin in serum, not in plasma. Besides, previous to compare biochemical results from the diverse studies, we should also consider differences on the recruited population, based on the SAH severity degree according to the clinical scales. Thus, Chou et al. study had almost 70% SAH patients with I–III HH score, while in the present work just 40% SAH patients had I–III HH grade.
Studies performed in animal models have suggested that gelsolin has a neuroprotective role, correlating its concentration to the cerebral infarct size.9 In an attempt to explain the differences between our results and the previously published concerning gelsolin levels in SAH patients, we hypothesize that the increase in gelsolin levels that we observe at admission is a reply of the organism to the primary damage, in an attempt to increase the gelsolin blood pool to scavenge and neutralize circulating actin and lipopolysaccharide that would exacerbate the pro-inflammatory response.10 In reference to our correlation results, we observe that higher gelsolin concentration is associated with lower GCS score and with higher HH classification, that is, the most severe the aSAH patients is, the higher gelsolin concentration. These results agree with the fact that aSAH patients had higher gelsolin levels than healthy controls.
In conclusion, our preliminary results indicate that, gelsolin serum levels increase at the moment of the bleeding in patients suffering aSAH and decrease within 48h, reaching healthy control levels. Moreover, 48-hour gelsolin serum concentration correlates with aSAH patient severity. We consider it would be of great interest to enlarge the sample size and the follow-up period to analyze the potential role of gelsolin as predictor of aSAH main complications (cerebral vasospasm and delayed cerebral ischemia) as well as patients’ outcome.
FundingThis study was funded by a grant from Consejería de Igualdad, Salud y Políticas Sociales de Andalucía, Spain (PI-0136-2012).
Conflict of interestThe authors declare no conflicting interest in this work.