The most used vasopressors in critically ill patients are exogenous catecholamines, mainly norepinephrine. Their use can be associated with serious adverse events and even increased mortality, especially if administered at high doses. In recent years, the addition of vasopressin has been proposed to counteract the deleterious effects of high doses of catecholamines (decatecholaminization) with the intention of improving the prognosis of these patients. Currently, vasopressin has two main indications: septic shock and vasoplegic shock in the postoperative period of cardiac surgery. In septic shock, current evidence favors its early initiation before reaching high doses of norepinephrine. In the postoperative period of cardiac surgery, the different benefits of the use of vasopressin have been studied, especially in patients with atrial fibrillation and pulmonary hypertension. When used properly, vasopressin is a safe an effective drug for the indications described above.
Hasta el momento, los vasopresores más utilizados en los pacientes críticos son las catecolaminas exógenas, fundamentalmente la noradrenalina. Sin embargo, estas pueden tener efectos secundarios graves e incluso pueden aumentar la mortalidad, sobre todo si se administran a dosis elevadas. En los últimos años se ha propuesto la adición de la vasopresina para contrarrestar sus efectos deletéreos (decatecolaminización) con la intención de mejorar el pronóstico de estos pacientes. Actualmente, la vasopresina tiene dos indicaciones principales: el shock séptico y el shock vasopléjico en el postoperatorio de cirugía cardiaca. En el shock séptico, la evidencia actual favorece el inicio temprano de vasopresina antes de alcanzar dosis elevadas de noradrenalina. En el postoperatorio de cirugía cardiaca se han estudiado los diferentes beneficios del uso de vasopresina, sobre todo en los pacientes con fibrilación auricular e hipertensión pulmonar. El uso correcto de la vasopresina lo hace un fármaco generalmente seguro y eficaz para estas indicaciones.
The hemodynamic resuscitation of shock requires measures aimed at increasing oxygen transport to restore adequate tissue perfusion.1,2
In this initial resuscitation process, along with antimicrobial therapy and infection source control, the administration of fluids and vasopressor drugs is essential. Catecholamines are the most widely used vasopressors, although they can have severe side effects and may even increase mortality at high doses.3,4 On the other hand, in patients with septic shock, their effectiveness can decrease due to changes to the adrenergic receptor or the action of various inflammatory mediators.5,6 Consequently, there is growing interest in the search for new vasopressor drugs that offer greater safety and efficacy in this hemodynamic resuscitation process. In recent years, the addition of vasopressin has been proposed to reduce the dose of catecholamines and improve the prognosis of critically ill patients.7
The objective of this document is to provide brief and precise information on ten key aspects of vasopressin that may be useful in the routine clinical practice of critical health care professionals.
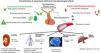
Characteristics of vasopressinVasopressin, also known as antidiuretic hormone (ADH), is a nonapeptide produced physiologically by the supraoptic and paraventricular nuclei of the hypothalamus. Initially produced as a prehormone from a gene on chromosome 20 p13, it is stabilized by neurophysin II in the Golgi apparatus, forming secretion granules that are transported by axons to the axonal terminals in the neurohypophysis, where they are stored as Herring bodies. Upon receiving an action potential, depolarization of these terminals occurs, with the opening of calcium channels and vesicular exocytosis, releasing vasopressin into the bloodstream. Its elimination half-life is approximately 10 minutes.8,9
The stimuli that can activate the production and release of vasopressin are fundamentally three (Fig. 1). First, the increase in plasma osmolarity, detected by osmoreceptors in the third ventricle anterior hypothalamus and anterior wall; a 1% change in osmolarity is sufficient to stimulate secretion. Second, a reduction in plasma volume. Volume receptors, located in the atria and at the junction to pulmonary veins and juxtaglomerular apparatus, detect decreases > 5% and send information via vagus nerve. The stimulation generated by the reduction in blood volume on vasopressin secretion is greater than the one caused by the increase in osmolarity. Finally, a decrease in mean arterial pressure also stimulates ADH secretion. In this case, receptors (baroreceptors) are located in the so-called pressure system (carotid, aortic, and ventricular receptors), which activate in response to decreases > 10%, sending information through the sensory branches of the glossopharyngeal and vagus nerves.
Other factors that can stimulate vasopressin production include stress, pain, nausea, hyperthermia, cholinergic agonists, nicotine, angiotensin II, or interleukin-1; there are also factors that can suppress it, such as hypothermia, high cortisol levels, alpha-adrenergic agonists, alcohol, opioids, or atrial natriuretic peptide, among others.10
Effects of vasopressinThe physiological effects of vasopressin result from its action on 3 main types of receptors:
- -
V1 or V1a: They mediate vasoconstriction. After binding, and through G proteins, they stimulate phospholipase C, which hydrolyzes phosphatidylinositol into inositol triphosphate (IP3), promoting the release of intracellular calcium and the contraction of vascular smooth muscle.
- -
V2: Their activation causes an antidiuretic effect. Upon binding, through G proteins, they stimulate adenylate cyclase, increasing cAMP levels and activate protein kinase A. This, in turn, recruits aquaporin-2 to the luminal membrane of the renal tubule, allowing water reabsorption.
- -
V3 or V1b: These are pituitary receptors that have a central effect, increasing ACTH through the activation of different G proteins and the increase of cAMP.8
Additionally, vasopressin can produce effects on pulmonary circulation (vasodilation), renal function (increasing renal perfusion and glomerular filtration by causing vasodilation in the afferent glomerular arterioles and vasoconstriction in the efferent arterioles through its action on V2 receptors), and blood coagulation (releasing factor VIII and von Willebrand factor).8
Vasopressin levels in shockA few studies have reflected on the changes that can occur in vasopressin levels during different phases of shock. Lin et al.11 demonstrated that patients in the initial stages of sepsis had mean blood vasopressin levels > 10.6 pg/mL, while in patients with established septic shock, these levels dropped down to 3.6 pg/mL. Landry et al.12 found similar vasopressin levels in patients with septic shock (3.1 pg/mL), while in other types of shock, such as cardiogenic shock, levels were much higher (22.7 pg/mL) and sustained over time. These inappropriately low plasma vasopressin levels in patients with septic shock are thought to be due to changes in baroreceptor-mediated vasopressin secretion. The administration of exogenous vasopressin provides the expected plasma concentration for the degree of hypotension with a marked pressor response in these patients.12 These results indicate that low endogenous hormone levels in septic shock contribute to sepsis-induced vasodilation.
Indications for vasopressinCurrently, vasopressin has 2 main indications based on scientific evidence: septic shock and vasoplegic shock in the postoperative period of cardiac surgery.13 However, in Spain, Empressin® is only indicated for septic shock,14 while Vasostrict® (commercial name in other countries) is indicated for the treatment of vasoplegic shock, regardless of the etiology.
Vasopressin in the treatment of septic shockPatients with septic shock present a classic hemodynamic profile of vasoplegia with increased vascular permeability, hypovolemia, and microcirculation changes.15 Additionally, numerous clinical and experimental studies have evidenced the presence of transient biventricular systolic and diastolic myocardial dysfunction.16,17 This dysfunction can be masked and undetected in a conventional study due to the decreased afterload due to vasoplegia, allowing the maintenance of normal ejection fraction and cardiac output (if adequate volume replacement has been performed).17 This hidden dysfunction could be detected by more advanced echocardiographic methods such as tissue Doppler or strain.18
The ultimate goal of hemodynamic resuscitation in any type of shock is to restore oxygen delivery to the tissues based on their metabolic needs. This resuscitation requires achieving a minimum mean arterial pressure (MAP) and adjusting oxygen transport to normalize metabolic parameters, such as plasma lactate, central (SvcO2) and mixed (SvO2) venous saturation, and regional perfusion parameters, such as capillary refill time (CRT).1,2
In this resuscitation process, the administration of fluids and vasopressor drugs constitutes the cornerstone of treatment. So far, the most widely used vasopressors are exogenous catecholamines, primarily norepinephrine. Although these drugs are useful in hemodynamic stabilization, they can have serious side effects and even be associated with increased mortality, especially if administered at high doses.19,20
In fact, in septic shock, there are already elevated concentrations of endogenous catecholamines (adrenaline and norepinephrine),21 which are necessary to counteract the cardiovascular effects of sepsis, such as vasoplegia and myocardial depression. These are detrimental phenomena when they occur severely and for long periods of time.22 Supraphysiological levels of endogenous or exogenous catecholamines are associated with poor adaptation in physiological stress states and increased energy expenditure, reduced splanchnic perfusion and intestinal immunogenicity, hepatic dysfunction, immunosuppression, and increased mortality.23
Besides their side effects, another drawback of catecholaminergic drugs in shock is that their action in patients with septic shock can be reduced due to downregulation of alpha and beta-adrenergic receptors caused by inflammatory mediators and high doses of catecholamines.4,5 This phenomenon can be associated with catecholamine-refractory shock, in which increasing vasopressor drugs does not restore adequate tissue perfusion, persisting hypotension, and hypoperfusion in the absence of hypovolemia.
Recently, strategies have been proposed to reduce the amount of catecholamines to counteract their deleterious effects, known as decatecholaminization.24 The possibility of adding non-adrenergic vasoconstrictor drugs such as vasopressin opens the discussion on how to define norepinephrine-refractory septic shock. Doses > 0.5 mcg/kg/min of norepinephrine (calculated by actual weight in BMI < 30 or by height-adjusted weight in BMI > 30)25 are associated with more adverse effects without adding clinical benefit, so this value has been suggested as the maximum dose or cutoff to define adrenergic-refractory shock.26 Other decatecholaminization strategies include adding corticosteroids to improve cardiac and vasopressor response or using analgesia and sedation to counteract the release of endogenous catecholamines. In fact, corticosteroids restore vascular sensitivity of alpha-agonist receptors within minutes or hours through non-genomic effects, with a corresponding increase in MAP and systemic vascular resistance.27 The latest Surviving Sepsis Campaign recommendations suggest the IV administration of hydrocortisone 50 mg every 6 hours or in continuous infusion when norepinephrine dose reaches ≥ 0.25 mcg/kg/min.13
The Surviving Sepsis Campaign 2021 recommendations suggest adding vasopressin in adult patients with septic shock and inadequate MAP values despite norepinephrine doses in the range of 0.25–0.5 mcg/kg/min instead of further increasing norepinephrine dose.13
This weak recommendation and moderate quality of evidence are based primarily on the VASST28 and VANISH29 clinical trials, and on an internal meta-analysis of clinical guidelines analyzing 10 controlled clinical trials. The VASST trial analyzed the effects of low-dose vasopressin (0.01–0.03 IU/min) as a norepinephrine-sparing agent, so it should not be understood as a study assessing vasopressin in catecholamine-refractory shock patients. This study did not show a significant improvement in 28-day mortality. However, a subgroup analysis found that patients with less severe shock requiring norepinephrine < 15 mcg/min had lower mortality rates when vasopressin was added (26.5% vs 35.7%; p = 0.05). From this study, it was inferred that adding low-dose vasopressin to patients with septic shock allows for a rapid reduction in norepinephrine dose and may reduce mortality in patients with a less severe profile. On the other hand, the VANISH trial was designed to assess whether early use of vasopressin at doses up to 0.06 IU/min could improve renal prognosis vs norepinephrine in patients with septic shock, as well as to assess the role of hydrocortisone. Patients with septic shock were randomized to receive vasopressin or norepinephrine in a 2 × 2 factorial design, analyzing acute kidney injury-free days. Although compared with norepinephrine, the early use of vasopressin did not significantly improve the number of acute kidney injury-free days, the vasopressin group had a lower need for renal replacement therapy initiation. Finally, the internal meta-analysis of the Surviving Sepsis Campaign that analyzed 10 randomized clinical trials found an improvement in mortality with the adjunctive use of vasopressin (RR, 0.91; 95% CI, 0.83–0.99).13
The optimal dose of vasopressin varies according to the indication and response to treatment. Following the VASST trial scheme, the recommended initial dose is 0.01 IU/min, which can be increased up to 0.03 IU/min, even up to 0.06 as used in the VANISH trial. Its combination with hydrocortisone can improve outcomes.30,31
To date, there are no clear recommendations on the most appropriate timing to initiate vasopressin. While the Surviving Sepsis Campaign13 recommends starting it when norepinephrine dose reaches 0.25 mcg/kg/min, the current trend is to start it much earlier, especially in patients requiring rapid increases in norepinephrine dose.32 Furthermore, recent studies suggest that mortality may be lower when vasopressin is initiated with equivalent norepinephrine doses of 10 mcg/min or with lactate concentrations < 2.3 mmol/L.33
Vasopressin in postoperative cardiac surgeryThis indication stems from the results of the multicenter, double-blind, randomized clinical trial (RCT) VANCS.34 The aim of this study was to reduce severe complications, including mortality, within 30 days of cardiac surgery. A total of 300 patients who developed vasoplegic shock (MAP < 65 mmHg after adequate crystalloid resuscitation (2.4 L on average) and a cardiac index > 2.2 L/m2) after cardiac surgery were randomized to receive norepinephrine (10–60 mcg/min) or vasopressin (0.01–0.06 IU/min). The primary endpoint (mortality plus severe complications within 30 days postoperatively) was achieved in 49% of patients on norepinephrine and 32.2% of those on vasopressin. A total of 49% of patients treated with norepinephrine experienced severe complications vs 32.2% in the vasopressin group [adjusted OR, 0.52 (0.36–0.75); p = 0.0005], primarily due to lower acute kidney injury [adjusted OR, 0.26 (0.15–0.46); p < 0.0001] in the vasopressin group. Also, although vasopressin-treated patients had a lower incidence of atrial fibrillation [adjusted OR, 0.37 (0.22–0.64); p = 0.0004], no significant differences were reported between the 2 groups in terms of ischemic adverse effects. A subsequent meta-analysis of 8 studies obtained similar results.35
Following these results, a subsequent consensus document on vasopressor treatment in cardiac surgery36 recommends the use of vasopressin in vasoplegia in these patients, especially in the presence of atrial fibrillation (strong recommendation, moderate evidence level). It also recommends it in cases of pulmonary hypertension (weak recommendation, very low evidence level), based on experimental results demonstrating a vasodilatory effect on the pulmonary artery at low doses (0.01–0.03 IU/min).37,38
Vasopressin in cardiopulmonary resuscitationVasopressin combined with methylprednisolone may have benefits in in-hospital cardiopulmonary resuscitation (CPR), as it has been confirmed that both during and after CPR, cortisol levels are low.35 Three RCTs that randomized patients in in-hospital cardiac arrest39–41 and a subsequent meta-analysis42 demonstrated that patients randomized to receive, after the first adrenaline dose, 20 U of vasopressin with 40 mg of methylprednisolone had a higher percentage of return of spontaneous circulation and a lower incidence of subsequent acute kidney injury vs patients on placebo. A subsequent subanalysis of a study by Andersen et al.37 on functional recovery at 6 months and 1 year, assessed using the Cerebral Performance Category (CPC) scale,43 did not show any improvement, yet the study had a very low statistical power. The latest European CPR clinical guidelines (2021) do not recommend the use of vasopressin in CPR.44 The results of randomized clinical trials (RCTs) were published after these guidelines. Pending the updated publication of the European resuscitation guidelines and with evidence from the RCT by Andersen et al.37 and the meta-analysis by Saghafi et al.,38 it could be recommended to combine 20 U of vasopressin and 40 mg of methylprednisolone after the first adrenaline dose during in-hospital CPR to achieve a higher proportion of patients recovering spontaneous circulation. To evaluate the effect of vasopressin on CPC, new RCTs with sufficient statistical power should be conducted.
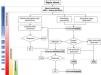
Hemodynamic monitoring during vasopressin useIn patients with septic shock, hemodynamic monitoring requires individualized adaptation based on the shock phase, treatment response, and specific patient characteristics. Recently, 4 phases of septic shock have been identified: Salvage, Optimization, Stabilization, and De-escalation.45 Each phase demands different monitoring techniques and resuscitation objectives (Table 1 and Fig. 2).
Phases of septic shock showing goals and proposed monitoring for each phase.
| Phase | Purpose | Goal | Intervention | Monitoring |
|---|---|---|---|---|
| Salvage | • Identify shock • Rule out cardiogenic component | • Mean Arterial Pressure • Optimize CRT | • Fluids • Norepinephrine | • Non-invasive blood pressure • Lactate • CRT • Echocardiography |
| Initial Optimization | • Optimize tissue perfusion | • Normalize CRT • Optimize Mean Arterial Pressure • Optimize CO | • Fluids according to response• Norepinephrine • Inotropes according to CO and echocardiography | • CRT • Lactate • If CVC: SvcO2• ΔPCO2• Internal calibration CO • Volume response evaluation• Echocardiography |
| Post-Optimization | • Optimize tissue perfusion | • Normalize tissue perfusion indices • Optimize Mean Arterial Pressure • Optimize CO | • Fluids according to response• Norepinephrine • Vasopressin • Inotropes according to CO and echocardiography | • CRT • Lactate • SvcO2• ΔPCO2• TPTD or PAC • Volume response evaluation • Echocardiography |
| Stabilization | • Support organ dysfunction • Minimize complications | • Preserve organ perfusion• Limit exposure to fluids, vasopressors, and inotropes • Limit accumulated volume | • Avoid fluids • Consider decongestion • Minimal dose of vasopressors and inotropes | • Maintain existing monitoring• ELWI if TPTD • Echocardiography • Lung ultrasound • Venous congestion |
| De-escalation | • Reduce initiated therapies while avoiding deterioration of tissue perfusion | • Negative fluid balance • Gradual withdrawal of vasopressors and inotropes • Preserve tissue perfusion | • Fluid removal via diuretics and/or mechanical means • Withdrawal of vasopressors and inotropes | • Blood pressure • Minimal CO monitoring • Volume response evaluation • Assess tissue perfusion • Lactate |
CRT, capillary refill time; CO, cardiac output; CVC, central venous catheter; ScvO2, central venous oxygen saturation; ΔPCO2, venous-to-arterial difference of carbon dioxide difference; TPTD, transpulmonary thermodilution; PAC, pulmonary artery catheter; ELWI, extravascular lung water index.
Monitoring sequence based on the response to hemodynamic treatments. 1: Associated cardiovascular comorbidity; 2: MAP ≥ target and without symptoms or signs of hypoperfusion; 3: MAP ≤ target and/or symptoms or signs of hypoperfusion; 4: If left ventricular systolic dysfunction is identified. 5: Having ruled out cardiac dysfunction. MAP, mean arterial pressure; CO, cardiac output; TPTD, transpulmonary thermodilution; PAC, pulmonary artery catheter.
During this stage, septic shock must be detected, and initial treatment, including volume replacement and norepinephrine, should be applied. Basic monitoring should include measuring blood pressure, heart rate, and physical examination.15
Early identification of clinical signs of hypoperfusion is essential, as hypotension is not always a definitive indicator of shock due to compensatory vasoconstriction that can maintain blood pressure even when tissue perfusion and oxygenation are significantly reduced.15
Blood lactate concentration measurement is useful for assessing tissue perfusion impairment. Based on studies correlating lactate dynamics and mortality, the Surviving Sepsis Campaign guidelines recommend monitoring hemodynamic resuscitation by repeatedly measuring blood lactate levels every 2 to 4 hours until coming back to normal levels.13 Recently, a capillary refill time (CRT)-guided early resuscitation strategy guided has proven capable of producing better results vs a lactate-guided strategy.46 CRT has been correlated with mortality and can be useful for monitoring fluid response, with a resuscitation target of CRT values < 3 seconds.47 Nonetheless, CRT does not identify the cause of tissue hypoperfusion.
During this phase, in case of lack of response to initial treatment or a history of cardiac dysfunction, guidelines recommend bedside echocardiography,15 as this non-invasive imaging modality allows us to estimate cardiac output and identify the cause of low output.48
Optimization phaseIn this phase, the goal is to optimize tissue perfusion by adjusting perfusion pressure and cardiac output. For patients who are unresponsibe to initial treatment and continue with tissue hypoperfusion, advanced hemodynamic monitoring should be considered to assess volume response, cardiovascular function, and cardiac output.15 This will allow adjusting preload in volume-responsive patients, afterload by adding vasopressin to norepinephrine treatment, or contractility using inotropes.
There are different reliable tools to measure cardiac output, and the choice of appropriate technique should be based on specific patient variables. In patients without comorbidities and minimal organ dysfunction, uncalibrated or internally calibrated cardiac output monitoring devices can be used.49 On the other hand, patients with associated comorbidities (e.g., coronary artery disease), or severe organ dysfunction (especially acute respiratory distress syndrome) can benefit from multimodal monitoring using transpulmonary thermodilution (TPTD) techniques or, in some cases, a pulmonary artery catheter (PAC) along with serial echocardiography.15
TPTD combines calibrated cardiac output measurement, precise beat-to-beat stroke volume estimation, static preload indicators, cardiac function indices, and estimates of extravascular lung water and vascular permeability. In this regard, TPTD is useful not only for predicting and monitoring volume response but also for providing information on the risks associated with its administration.50
By measuring cardiac output, the PAC, pulmonary pressures, and mixed venous oxygen saturation, allows evaluating cardiac output adequacy and its determinants. Although it does not predict volume response, the PAC is useful for assessing fluid efficacy and tolerance. Simultaneous measurement of central venous pressure and pulmonary artery pressure makes the PAC ideal for monitoring patients with right heart dysfunction.51
Stabilization and de-escalation phasesDuring these stages, the goal of hemodynamic support shifts from optimizing tissue perfusion to preventing associated complications, minimizing exposure to volume, vasopressors, and inotropes.52
In this phase, cardiac dysfunction may persist, and side effects related to volume overload induced by initial resuscitation may appear. Therefore, previously implemented monitoring systems can continue to be used. Monitoring of extravascular lung water, lung ultrasound, and evaluation of portal, renal, and hepatic venous flow patterns via ultrasound can help identify signs of congestion and indicate the need for volume withdrawal.53
On the other hand, for vasopressor de-escalation, evaluating dynamic arterial elastance, calculated using the pulse pressure variation (PPV) to stroke volume variation (SVV) ratio, can be useful. This evaluation can predict hypotension onset and identify patients who are eligible for vasopressor reduction.54
Safety in vasopressin administration in critically ill patientsAmong vasopressors used in routine clinical practice, vasopressin has the highest vasoconstrictor capacity in experimental animal models.55
This potent vasoconstrictive action is related to the most severe adverse events in critically ill patients and has questioned its use in this type of patient for a long time.
The use of high-dose vasopressin (understood as doses > 0.04–0.05 IU/min) has been associated with decreased cardiac output in septic patients and significant ischemic complications such as hepatic, mesenteric, digital, or lingual hypoperfusion,56–58 especially in obese patients or those with baseline atherosclerotic disease.56
Despite these severe complications, low-dose vasopressin administration (0.01 to 0.04 IU/min) has proven, as mentioned earlier, effective in treating septic shock or distributive shock in postoperative cardiac surgery with persistent hypotension despite norepinephrine28,59,60 without being associated with an increased incidence of cardiovascular complications (e.g., decreased cardiac output or arrhythmias),59–61 or ischemic complications at any level (splenic, coronary, digital, or cerebrovascular).28,34,60
Therefore, it could be stated that low-dose vasopressin administration in patients with septic shock or distributive shock post-cardiac surgery with hypotension despite norepinephrine at 0.2 mcg/kg/min and adequate crystalloid resuscitation is safe and effective. In obese patients, those with previous atherosclerotic disease, or elderly patients with cardiovascular risk factors, vasopressin use should along with closer monitoring to detect early possible ischemic adverse events.
Vasopressor weaning or vasopressin discontinuationWhile there are currently available recommendations on when to start vasopressin as a second vasopressor for the management of septic shock, there are no established recommendations on how to discontinue or withdraw it.13
During recovery phase of shock, vascular tone recovery occurs, and vasopressors are gradually withdrawn. However, even in this phase, vasopressor discontinuation can produce significant hypotension with the risk of new organ failures.
Although the available literature is scarce on this topic, most studies seem to favor initial norepinephrine withdrawal rather than vasopressin, as early vasopressin withdrawal is often associated with a higher frequency of hypotension. Bauer et al.62 found that patients in whom vasopressin was initially withdrawn developed a significantly higher incidence rate of hypotension within the first 24 hours (55.6% vs 15.6%, p < 0.008), with a risk up to 5 times higher (RR, 5.9, 95% CI, 1.7–21). Similarly, other authors found that vasopressin withdrawal was associated with clinically significant hypotension, especially when withdrawn within the first 48 hours, and that this hypotension seemed to persist within the first 24 hours after withdrawal.63,64 No significant differences in mortality or the ICU stay were ever found. Two meta-analyses also confirm these results.65,66
Although these studies had limitations related to their retrospective nature, there is currently only 1 randomized clinical trial that seems to favor initial vasopressin withdrawal, showing a higher incidence rate of hypotension after norepinephrine withdrawal first (68.4% vs 22.5%, p < 0.005).67 However, this study only evaluated the first hour after vasopressor withdrawal and could not confirm the persistence of hypotension beyond this period. Therefore, these findings may be more related to the drug half-life per se rather than the effect generated after withdrawal. This same study showed that copeptin levels were significantly lower in patients who developed hypotension after vasopressin withdrawal, suggesting the usefulness of this marker to assess vasopressin deficiency. This study also did not demonstrate significant differences in mortality or the length of stay.
Therefore, current literature seems to favor norepinephrine withdrawal before vasopressin to avoid reactive hypotension, although the order of withdrawal of one or the other vasopressor does not seem to be associated with a higher mortality rate or with longer lengths of stay.
Table 2 illustrates the main practical considerations on the use of vasopressin in critically ill patients.
Main practical considerations for the use of vasopressin in critically ill patients.
| Patients who benefit from vasopressin administration: |
|
|
| Initial doses and progression of vasopressin: |
|
|
| Weaning vasopressors: |
|
|
|
MAP, mean arterial pressure; DBP, diastolic blood pressure.
Vasopressin is a drug studied as an alternative to high-dose catecholaminergic vasopressors. Different studies have demonstrated that, at low doses, it is an effective drug to maintain MAP goals in patients with septic shock and vasoplegic shock in postoperative cardiac surgery. Current medical practice should consider applying the favorable evidence on vasopressin use in critically ill patients and avoid the use of high doses of catecholaminergic vasopressors associated with deleterious effects and poor prognosis. Its use should be accompanied by necessary hemodynamic monitoring to optimize treatment. More studies are needed to expand indications in specific patient groups and optimize the management of combined vasopressor use when vasopressin is added to patient treatment.
FundingAOP Health facilitated the authors’ attendance at an in-person meeting. The rest of the work was conducted via email.
We wish to thank Dr. Erika P. Plata-Menchaca.