Despite resuscitation strategies, outcomes remain poor in cardiac arrest, with an in-hospital cardiac arrest (IHCA) survival rate of 15–20% and severe neurological deficits in 10–20% of survivors.1
ECPR (extracorporeal cardiopulmonary resuscitation) is the use of venoarterial ECMO in refractory cardiac arrest to maintain perfusion of vital organs. Recent guidelines2,3 recognize its potential benefit when compared to conventional cardiopulmonary resuscitation (CPR).
We report our experience during the first year of implementation of an ECPR program for IHCA.
We performed a retrospective analysis of prospectively collected data on all patients who underwent ECPR for refractory IHCA at Alvaro Cunqueiro University Hospital (Vigo, Spain) from November 2017 to November 2018.
Patients with refractory IHCA were eligible for ECPR if they had a witnessed arrest, CPR initiation within the first 5min, and a presumably reversible etiology.
Exclusion criteria were age more than 75 years, unwitnessed arrests, asystole (except in case of witnessed arrests and evident aetiologies) or significant comorbidities.
The ICU-CPR team is alerted when a patient arrests in our center, and starts advanced CPR upon arrival. The ECPR code leader is responsible for evaluating if the patient is a potential candidate for ECPR, and if so, ECPR code is activated.
The intensivist or cardiothoracic surgeon starts percutaneous echo-guided femorofemoral venoarterial cannulation after 15–20min of CPR. The Cardiohelp device (Maquet, Getinge, Germany) is used in all cases. An unfractionated heparin bolus (50–100IU/kg) is administered during cannulation followed by an infusion.
After ECMO initiation, therapeutic temperature management is initiated (36°C), and the patient is transferred to the catheterization laboratory or operation theater depending on the arrest etiology. A 7Fr backflow cannula is inserted to provide distal limb perfusion after completion of all interventions.
Patient data are collected as part of quality assurance protocols, and informed consent is obtained from a patient's relative. Data treatment was approved by the hospital ethics committee.
The primary outcome was functional favorable survival to hospital discharge, measured with cerebral performance category (CPC 1–2). Secondary outcomes include 1-month survival with a CPC score of 1–2, and complications.
Analysis was mainly descriptive. Baseline and follow-up categorical variables were summarized with frequencies and percentages and compared using the Fisher exact test. Baseline and follow-up continuous variables were summarized using the summary statistics n, median, and interquartile range (IQR). Statistical analyses were performed using SPSS (IBM, New York).
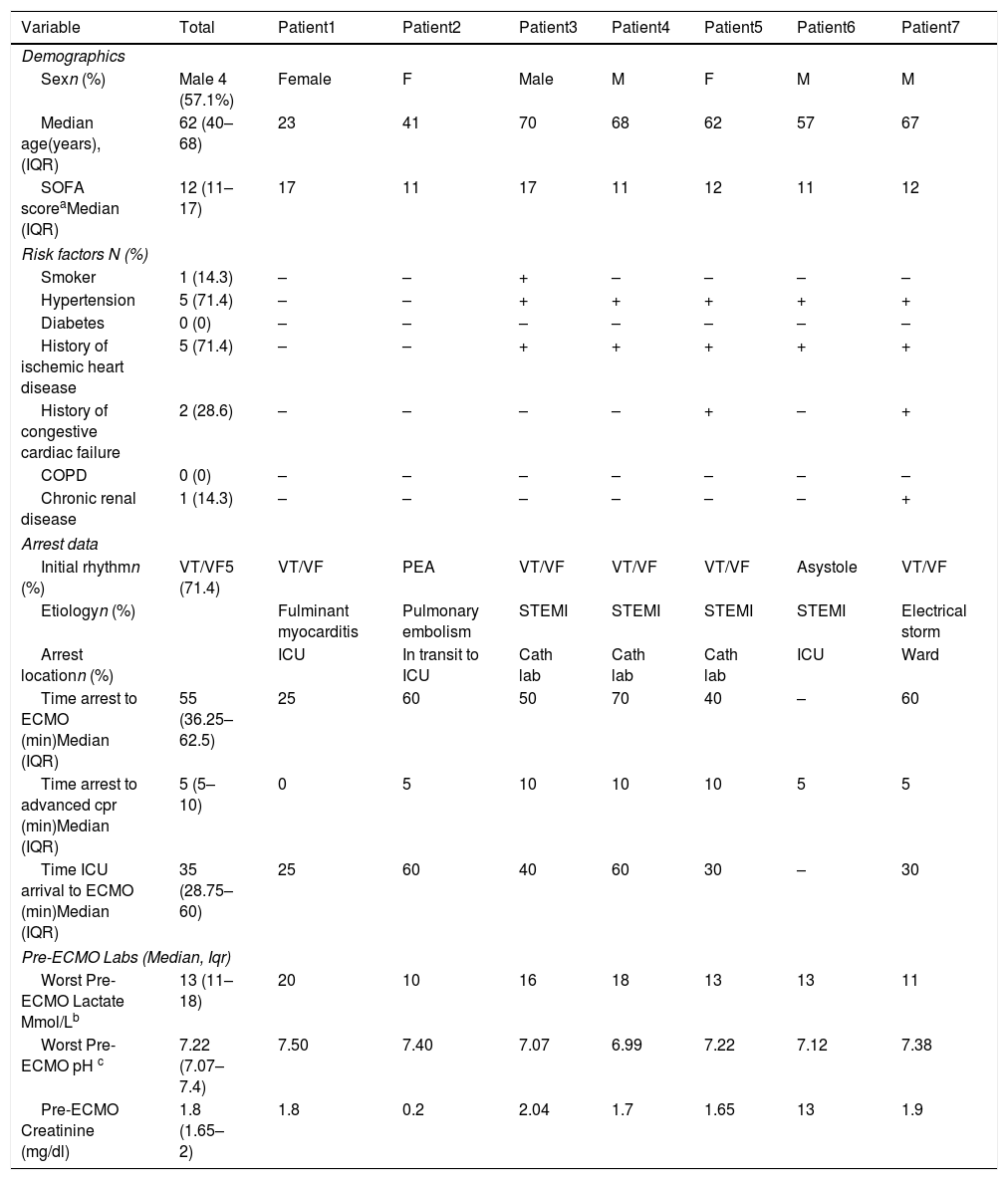
From November 2017 to November 2018, seven patients were included in the ECPR program (Table 1). Median age was 62 years (IQR 40–68 years). The main risk factors were arterial hypertension (71.4%) and a history of coronary artery disease (71.4%).
Demographics and arrest data.
| Variable | Total | Patient1 | Patient2 | Patient3 | Patient4 | Patient5 | Patient6 | Patient7 |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Sexn (%) | Male 4 (57.1%) | Female | F | Male | M | F | M | M |
| Median age(years), (IQR) | 62 (40–68) | 23 | 41 | 70 | 68 | 62 | 57 | 67 |
| SOFA scoreaMedian (IQR) | 12 (11–17) | 17 | 11 | 17 | 11 | 12 | 11 | 12 |
| Risk factors N (%) | ||||||||
| Smoker | 1 (14.3) | – | – | + | – | – | – | – |
| Hypertension | 5 (71.4) | – | – | + | + | + | + | + |
| Diabetes | 0 (0) | – | – | – | – | – | – | – |
| History of ischemic heart disease | 5 (71.4) | – | – | + | + | + | + | + |
| History of congestive cardiac failure | 2 (28.6) | – | – | – | – | + | – | + |
| COPD | 0 (0) | – | – | – | – | – | – | – |
| Chronic renal disease | 1 (14.3) | – | – | – | – | – | – | + |
| Arrest data | ||||||||
| Initial rhythmn (%) | VT/VF5 (71.4) | VT/VF | PEA | VT/VF | VT/VF | VT/VF | Asystole | VT/VF |
| Etiologyn (%) | Fulminant myocarditis | Pulmonary embolism | STEMI | STEMI | STEMI | STEMI | Electrical storm | |
| Arrest locationn (%) | ICU | In transit to ICU | Cath lab | Cath lab | Cath lab | ICU | Ward | |
| Time arrest to ECMO (min)Median (IQR) | 55 (36.25–62.5) | 25 | 60 | 50 | 70 | 40 | – | 60 |
| Time arrest to advanced cpr (min)Median (IQR) | 5 (5–10) | 0 | 5 | 10 | 10 | 10 | 5 | 5 |
| Time ICU arrival to ECMO (min)Median (IQR) | 35 (28.75–60) | 25 | 60 | 40 | 60 | 30 | – | 30 |
| Pre-ECMO Labs (Median, Iqr) | ||||||||
| Worst Pre-ECMO Lactate Mmol/Lb | 13 (11–18) | 20 | 10 | 16 | 18 | 13 | 13 | 11 |
| Worst Pre-ECMO pH c | 7.22 (7.07–7.4) | 7.50 | 7.40 | 7.07 | 6.99 | 7.22 | 7.12 | 7.38 |
| Pre-ECMO Creatinine (mg/dl) | 1.8 (1.65–2) | 1.8 | 0.2 | 2.04 | 1.7 | 1.65 | 13 | 1.9 |
M, Male; F, Female; COPD, Chronic Obstructive Pulmonary Disease; ECMO, Extracorporeal Membrane Oxygenation; IQR, Interquartile range.
SOFA (Sequential Organ Failure Assessment): worst SOFA score post-cardiac arrest. No difference was detected between survivors (12; IQR 11–17) and non-survivors (11.5; IQR 11–15.75); p=0.81.
The most frequent cause of cardiac arrest was ST-elevation myocardial infarction (STEMI) in four cases (57.1%). Shockable rhythm was the initial rhythm in five (71.4%) patients.
All the cardiac arrests were witnessed and involved bystander CPR in less than 1min. The median time from bystander CPR to advanced ICU CPR was 5min (IQR 5–10min). The median low-flow time was 55min (IQR 36.25–62.5min). The median low-flow time was lower in survivors (40min; IQR 25–60min) than in non-survivors (60min; IQR 50–70min), but the difference was not significant (p=0.191).
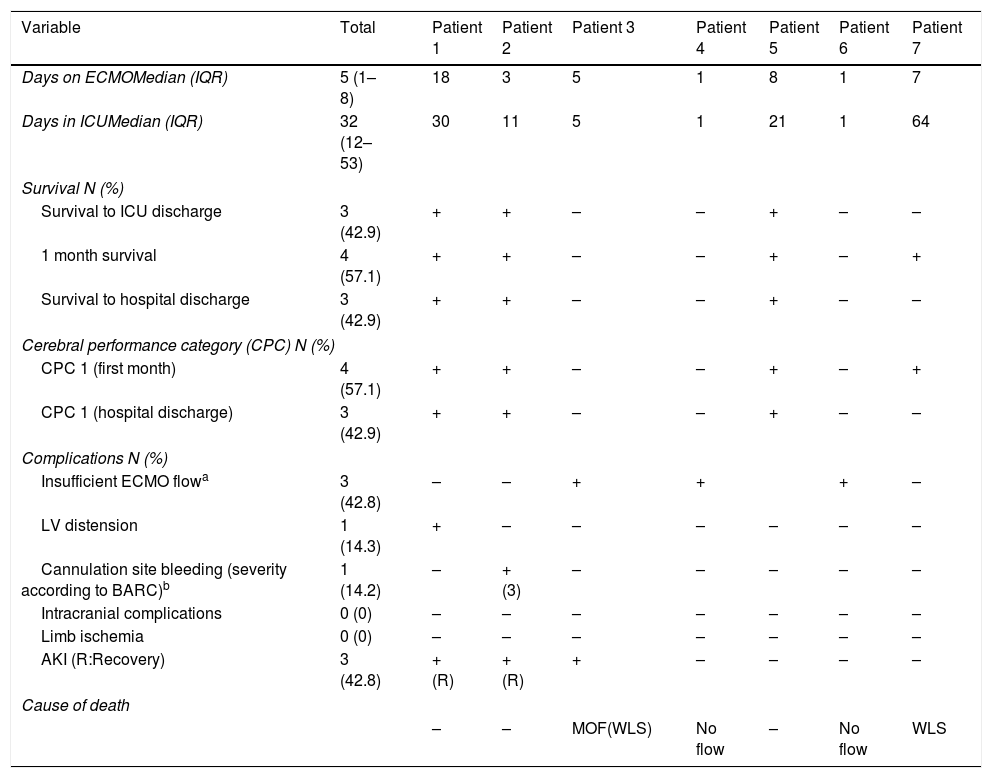
Table 2 shows the proceedings and outcome data. The median duration of ECMO support was 5 days (IQR 1–8 days). After commencement of ECMO, five patients (71.4%) underwent coronary angiography, three of whom underwent percutaneous coronary intervention.
ECMO proceedings and outcome data.
| Variable | Total | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|---|
| Days on ECMOMedian (IQR) | 5 (1–8) | 18 | 3 | 5 | 1 | 8 | 1 | 7 |
| Days in ICUMedian (IQR) | 32 (12–53) | 30 | 11 | 5 | 1 | 21 | 1 | 64 |
| Survival N (%) | ||||||||
| Survival to ICU discharge | 3 (42.9) | + | + | – | – | + | – | – |
| 1 month survival | 4 (57.1) | + | + | – | – | + | – | + |
| Survival to hospital discharge | 3 (42.9) | + | + | – | – | + | – | – |
| Cerebral performance category (CPC) N (%) | ||||||||
| CPC 1 (first month) | 4 (57.1) | + | + | – | – | + | – | + |
| CPC 1 (hospital discharge) | 3 (42.9) | + | + | – | – | + | – | – |
| Complications N (%) | ||||||||
| Insufficient ECMO flowa | 3 (42.8) | – | – | + | + | + | – | |
| LV distension | 1 (14.3) | + | – | – | – | – | – | – |
| Cannulation site bleeding (severity according to BARC)b | 1 (14.2) | – | + (3) | – | – | – | – | – |
| Intracranial complications | 0 (0) | – | – | – | – | – | – | – |
| Limb ischemia | 0 (0) | – | – | – | – | – | – | – |
| AKI (R:Recovery) | 3 (42.8) | +(R) | +(R) | + | – | – | – | – |
| Cause of death | ||||||||
| – | – | MOF(WLS) | No flow | – | No flow | WLS | ||
ECMO, Extracorporeal membrane oxygenation; ICU, Intensive Care Unit; IQR, Interquartile range; CPC, Cerebral Performance category; CPR, cardiopulmonary resuscitation; LV, left ventricle; AKI, Acute kidney injury; MOF, Multiorgan Failure; WLS, Withdrawal of life support.
Insufficient ECMO flow: An ECMO flow>1L/min could not be achieved in patients 3, 4 and 6 after ECMO initiation. Patients 3 and 4 suffered sternal fracture and mediastinal hematoma secondary to mechanical chest compressions, leading to insufficient ECMO flow (patient 4 died immediately. Flow could be restored in patient 3 after sternotomy, but he developed multiorgan failure). Patient 6 died immediately after ECMO initiation. Necropsy did not show vessel, cardiac or thoracic injury.
Three (42.9%) patients survived to hospital discharge, 100% of survivors with CPC score of 1. Four patients (71.4%) survived 1 month after cardiac arrest with a CPC score of 1 (patient 7 was finally withdrawn from life support after being considered not suitable for further procedures).
The main cause of death was an impossibility to achieve an adequate ECMO flow shortly after cannulation in 3 patients. No patients suffered intracranial complications or limb ischemia.
ECPR has emerged as a promising technique in refractory cardiac arrest. The rate of survival with favorable neurological outcomes after 16min of conventional CPR is reported to be less than 2%.4
Nevertheless, ECMO maintains organ perfusion, which could halt the accumulation of ischemic injury providing time to reverse the underlying etiology. A neurologically favorable survival has been observed in ECPR after prolonged CPR (25% survival with>50min of CPR and 19% with more than 60min) compared to 0% survival after 40min of conventional CPR.5
Although there is a lack of definite evidence, several recent studies6,7 and meta-analysis8 have shown promising results with ECPR compared to CPR, with neurologically favorable survival rates greater than 30%.
There are few reports of ECPR programs in Spain.9 Establishing an ECPR program requires thorough training, organization and protocolization. The key of a successful program is to reduce barriers to achieve quick deployment of ECMO.10 Three factors are paramount. First, rapid and individualized decision-making, with an ECPR leader in charge of activating ECPR code. Ideally, the CPR leader and ECPR leader should be different to optimize both procedures. An ECMO specialist is available 24h in our ICU, assuming the ultimate decision about activating ECPR code.
Second, a rapid team deployment is crucial to reduce no-flow and low-flow time, as they have been described as critical factors for survival. Quality practices should analyze time intervals to work specifically in each one to avoid unnecessary delays.
Finally, cannulation is subject to potentially devastating complications, especially if performed in an unfamiliar environment. In our case, all patients are transferred to the ICU for cannulation, except if they arrest in the catheterization laboratory.
Our experience suggests that an ECPR program is feasible and increase survival in selected patients. Given the high complexity of these patients, an ICU-based multidisciplinary approach may offer a better advantage in terms of covering the range of arrest aetiologies and the best synchronization of CPR and ECPR teams.
Our study has limitations. First, it is a single-center study. Furthermore, the small sample size and its retrospective nature make it underpowered to reach definite conclusions.
Conflict of interestNone.