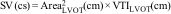Edited by: Ana Ochagavía - Hospital Universitario de Bellvitge. L'Hospitalet de Llobregat. Barcelona. Spain.
Last update: March 2024
More infoSurgical complexity as well as comorbidities in patients undergoing cardiac surgery is increasing. Early detection and management of post-surgical complications are key points to reduce morbidity and mortality. Ultrasound technique plays a main rol in cardiopulmonary, hemodynamic and etiological assessment of the critically ill, providing immediate, reliable and, sometimes, conclusive information, clarifying many clinical situations with an inappropiate therapeutic response. For all these reasons ultrasound is an essential diagnostic tool. In this chapter we will focus, mainly, on functional and hemodynamic assessment and on the detection of most common cardiological complications in the postoperative period after cardiac surgery.
La complejidad de los procedimientos quirúrgicos, así como la comorbilidad de los pacientes sometidos a cirugía cardiaca va en aumento. La detección y tratamiento precoz de las complicaciones postquirúrgicas son parte del éxito en la reducción de la morbimortalidad. La introducción de la técnica ecográfica ha sido fundamental en la valoración cardiopulmonar, hemodinámica y etiológica del paciente crítico, aportando información inmediata, fiable y a veces concluyente, permitiendo aclarar muchas situaciones clínicas sin respuesta terapéutica aceptable. Tratándose de una herramienta diagnóstica esencial. En este capítulo nos centraremos, fundamentalmente, en la valoración funcional, hemodinámica y en la detección de las complicaciones cardiológicas más frecuentes en el postoperatorio de cirugía cardiaca.
The complexity of surgical procedures, as well as the comorbidity of patients undergoing cardiac surgery (CS), is increasing. Early detection and treatment of postoperative complications are part of the success in reducing morbidity and mortality.
The main serious problems that can occur in the cardiac surgery immediate postoperative (IPO) are hemodynamic instability, severe respiratory failure, and/or states of low cardiac output (CO). Inappropriate or delayed management can lead to multiorgan dysfunction syndrome, the main cause of prolonged hospitalizations and morbidity and mortality.
The addition of the information provided regarding the surgical technique performed, perioperative events, preoperative imaging studies, along with clinical, semiological, analytical, and hemodynamic information at the time of evaluation, should help us identify the etiological cause, determine the patient’s hemodynamic profile, and the main component causing it, thus enabling the selection of initial treatment and assessing the degree of response to the decision made.
The introduction of ultrasound techniques1 (transthoracic echocardiography -TTE-, transesophageal echocardiography -TEE- and lung ultrasound -LU-) has been crucial in the cardiopulmonary, hemodynamic, and etiological assessment of critically ill patients, providing immediate, reliable, and sometimes conclusive information, allowing the clarification of many clinical situations without an acceptable therapeutic response.
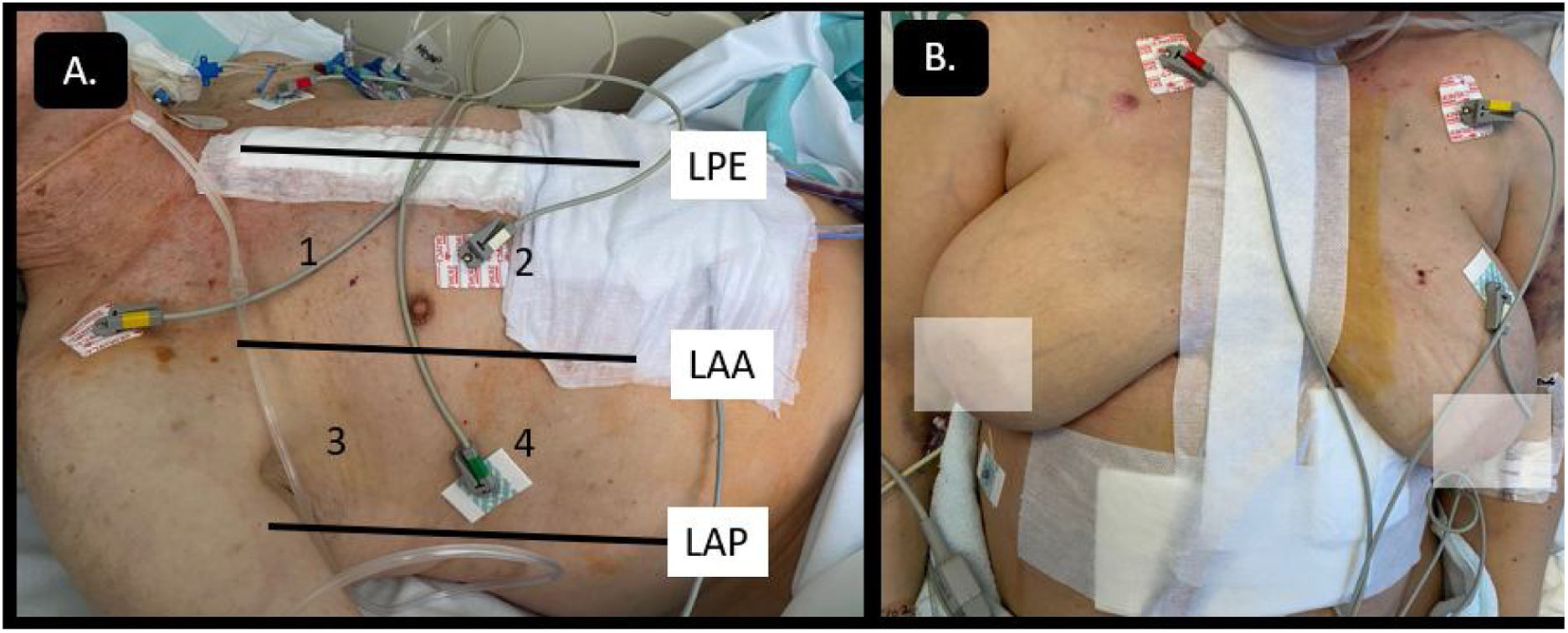
Cardiac surgery post-operated patients (CPO) pose certain technical difficulties associated with patient mobilization limitations, dressings, drains, inability to use the subxiphoid approach, postoperative pain, intubation, ventilatory support, etc. (Fig. 1). The technical limitations that hinder image acquisition, along with the frequent presence of loculated clots or pericardial-pleural effusion, could complicate the interpretation of ultrasound studies, sometimes requiring the combination of other imaging modalities for proper study.
Since respiratory assessment is addressed in another chapter of this series, we will focus especially on the study of the most common cardiovascular complications detected in the IPO.2
More useful projectionsCardiological studyIt is sometimes constrained by technical difficulties, which is why TEE should not be ruled out when the risk of delayed diagnosis is associated with worse prognoses. The examination should cover as many windows as possible and use lower-frequency transducers to achieve greater penetration, especially in obese patients. If the clinical situation allows it, tilting the patient slightly to the left could help.
The parasternal window and apical 4- and 2-chamber views provide comprehensive studies of the left ventricle (LV), as well as the transverse axes of aortic and mitral valves, allowing us to determine the presence of residual regurgitation or paravalvular leaks when properly aligned. Subxiphoid access provides an overall view of the right cavities, the size of the right ventricle (RV), tricuspid function, and the size of the inferior vena cava. However, it is highly conditioned by the presence of mediastinal and sternal drains.
Pulmonary studyBecause of the aforementioned anatomical limitations, it seems like a practical thing to do to conduct a modified study proposed by Volpicelli3,4 (Fig. 1A). For further information on this topic, the reader is referred to chapters 3 and 4 of this series.
Left and right ventricular functionThe assessment of left ventricular (LV) function is one of the most important parameters in echocardiographic studies in most cases due to its prognostic, diagnostic, and therapeutic implications. However, evaluating the LV function is challenging because there is no single measure that provides us with the big picture of cardiac function. It often requires using various echocardiographic modalities to complete the assessment.
The factors that determine the LV function include preload, afterload, intrinsic contractility, ventricular size, contraction frequency, electrical uniformity, and mechanical contraction (ventricular synergy and interdependence) and the rate of myocardial relaxation (lusitropy).5
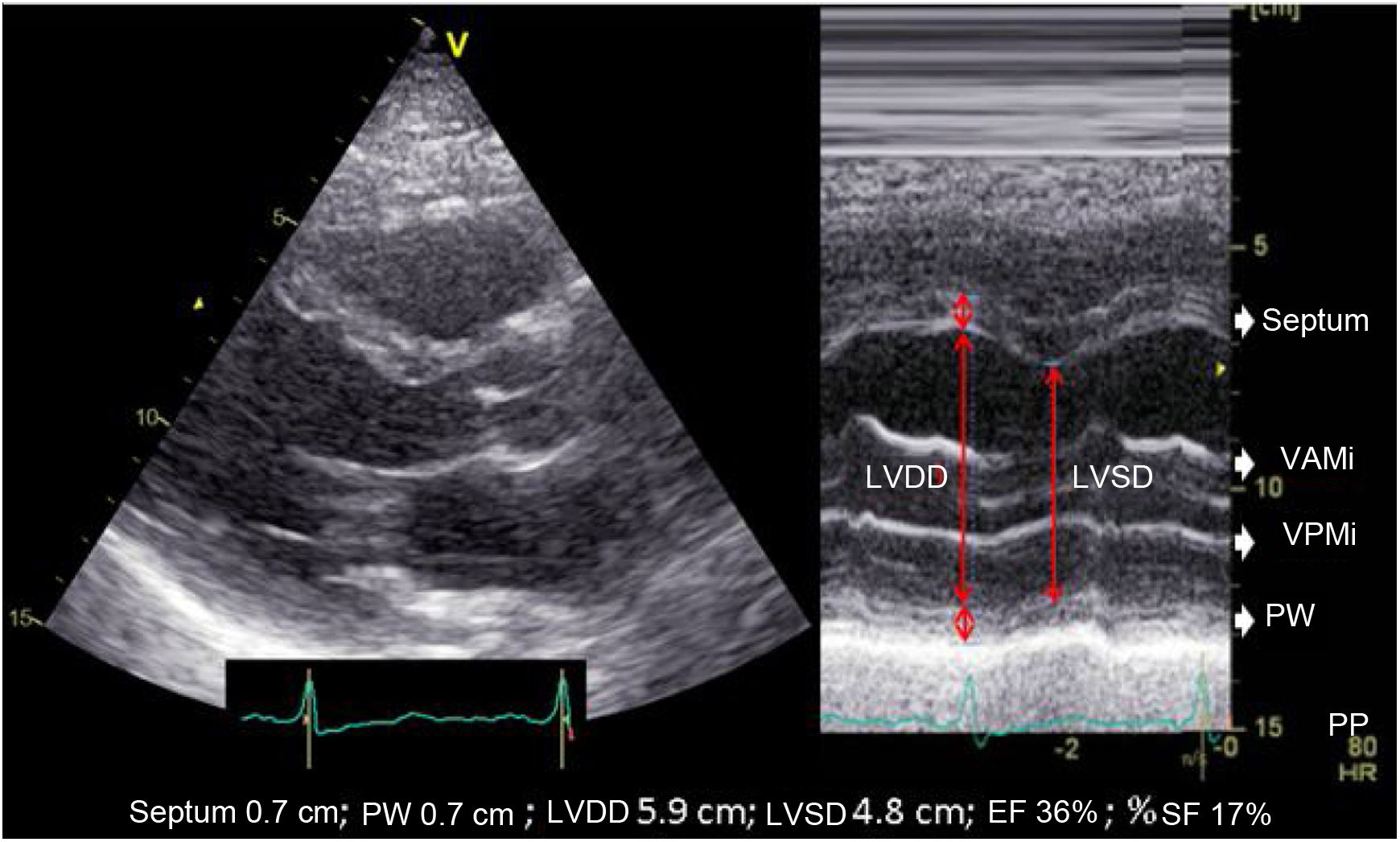
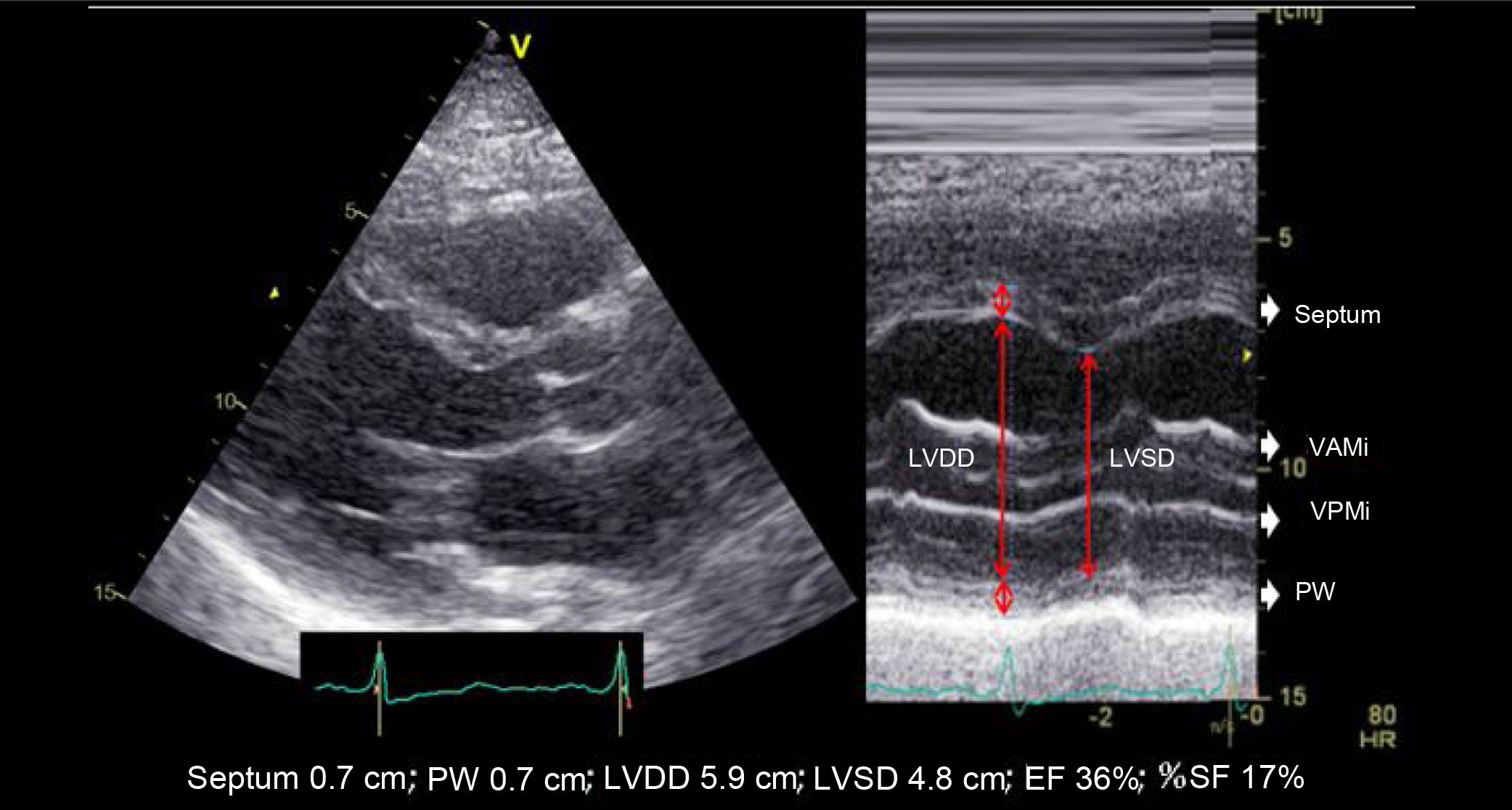
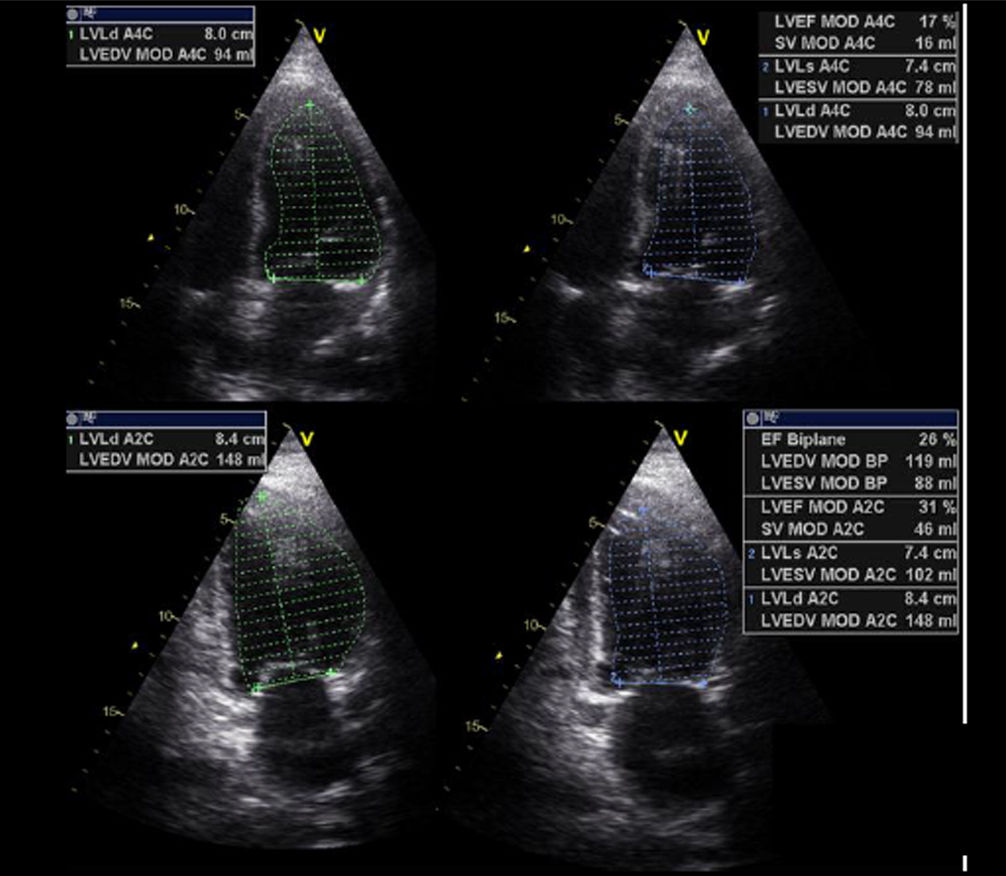
The most widely used echocardiographic parameter in the routine clinical practice is the left ventricular ejection fraction (LVEF), although it may not always adequately reflect ventricular function. Therefore, a comprehensive evaluation includes the analysis of diameters, volumes, geometry, mass, wall thickness, segmental contractility, diastolic function, and estimation of filling pressures. These aspects, however, go beyond the scope of this chapter (Table 1).6–10
Assessment of ventricular systolic function.
| GLOBAL SYSTOLIC FUNCTION | ||
| Visual estimation is the most widely used method to study the systolic function. While this method has acceptable correlation with ventriculography-determined function when performed by experienced observers, its accuracy decreases with less experience, as well as in cases of ventricles with abnormal morphology, non-homogeneous contraction, segmental contractility abnormalities, or in the presence of asynchrony. | ||
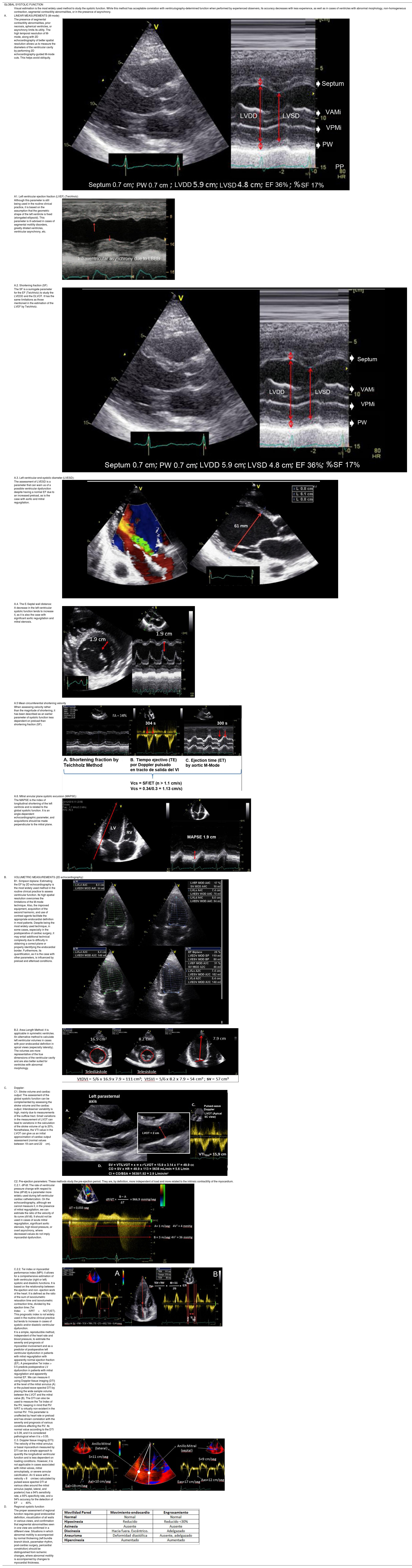
| A. | LINEAR MEASUREMENTS (M-mode) | |
| The presence of segmental contractility abnormalities, prior necrosis, spherical ventricles, or asynchrony limits its utility. The high temporal resolution of M-mode, along with 2D echocardiography of better spatial resolution allows us to measure the diameters of the ventricular cavity by performing 2D echocardiography-guided M-mode cuts. This helps avoid obliquity. | ||
| A1. Left ventricular ejection fraction (LVEF) (Teichholz) | ||
| Although this parameter is still being used in the routine clinical practice, it is based on the assumption that the geometric shape of the left ventricle is fixed (elongated ellipsoid). This parameter is ill-advised in cases of segmental motility disorders, greatly dilated ventricles, ventricular asynchrony, etc. | ||
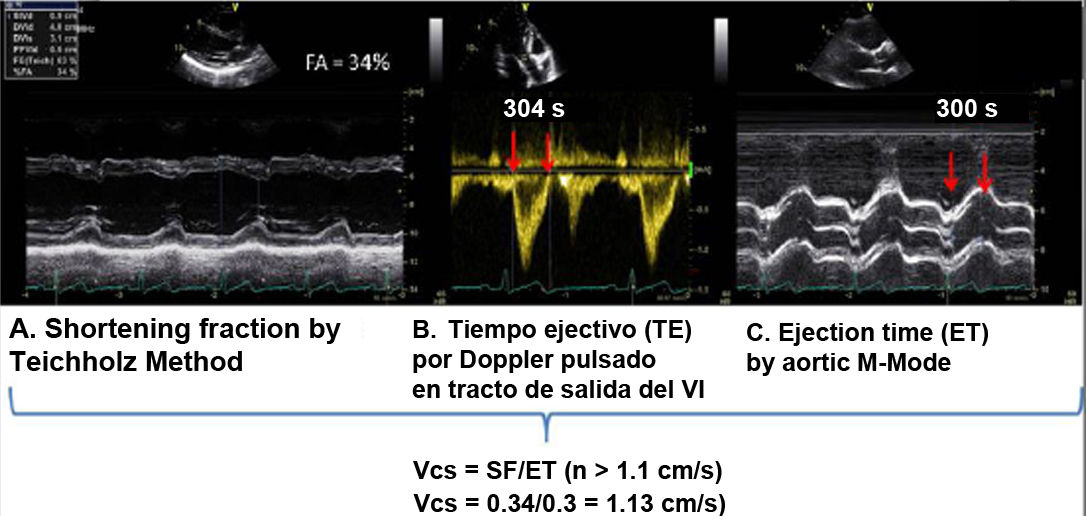
| A.2. Shortening fraction (SF) | ||
| The SF is a surrogate parameter for the EF (Teichholz) to study the LVEDD and the DLVOT. It has the same limitations as those mentioned in the estimation of the LVEF by Teichholz. | ||
| A.3. Left ventricular end-systolic diameter (LVESD): | ||
| The assessment of LVESD is a parameter that can warn us of a possible ventricular dysfunction despite having a normal EF due to an increased preload, as is the case with aortic and mitral regurgitation. | ||
| A.4. The E-Septal wall distance: | ||
| A decrease in the left ventricular systolic function tends to increase it, as it is also the case with significant aortic regurgitation and mitral stenosis. | ||
| A.5 Mean circumferential shortening velocity | ||
| When assessing velocity rather than the magnitude of shortening, it has been described as an earlier parameter of systolic function less dependent on preload than shortening fraction (SF). | ||
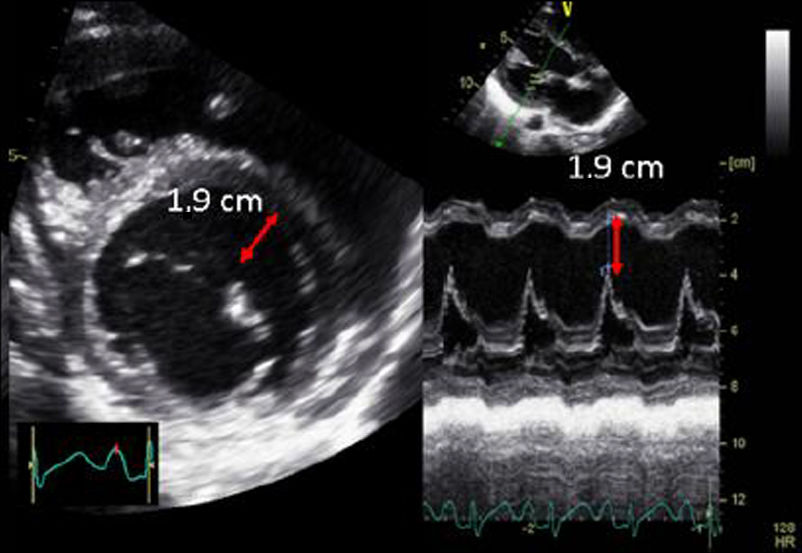
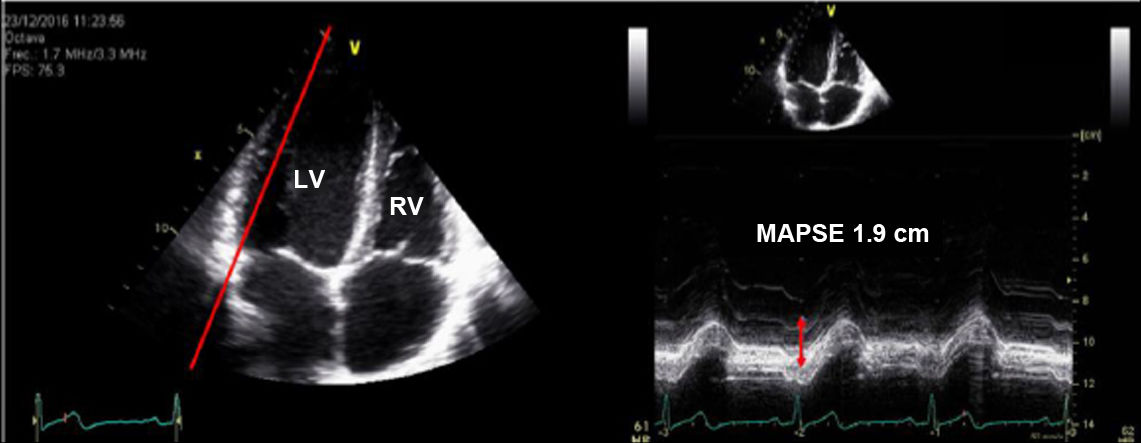
| A.6. Mitral annular plane systolic excursion (MAPSE): | ||
| The MAPSE is the index of longitudinal shortening of the left ventricle and is related to the global systolic function. It is an angle-dependent echocardiographic parameter, and acquisitions should be made perpendicular to the mitral plane. | ||
| B. | VOLUMETRIC MEASUREMENTS (2D echocardiography): | |
| B1. Simpson biplane: Estimating the EF by 2D echocardiography is the most widely used method in the routine clinical practice to assess ventricular function. Its high spatial resolution overcomes the limitations of the M-mode technique. Also, the improved equipment, acquisition of the second harmonic, and use of contrast agents facilitate the appropriate endocardial definition in most patients. Despite being the most widely used technique, in some cases, especially in the postoperative of cardiac surgery, it may entail additional technical complexity due to difficulty in obtaining a correct plane or properly identifying the endocardial border. Furthermore, its quantification, as it is the case with other parameters, is influenced by preload and afterload conditions. | ||
| B.2. Area-Length Method: it is applicable in symmetric ventricles. An alternative method to calculate left ventricular volumes in cases with poor endocardial definition in apical views (especially laterally). The volumes are more representative of the true dimensions of the ventricular cavity and are also better suited for ventricles with abnormal morphology. | ||
| C. | Doppler: | |
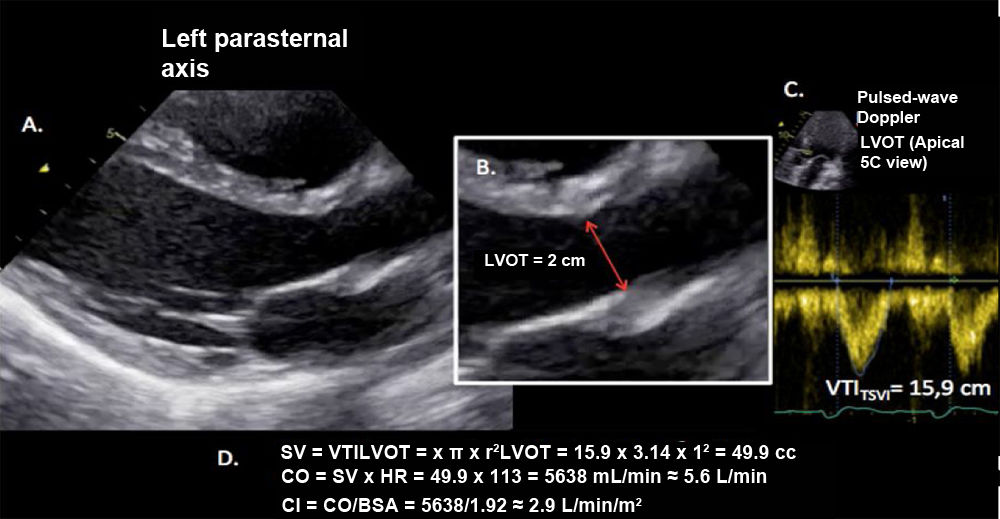
| C1. Stroke volume and cardiac output: The assessment of the global systolic function can be complemented by assessing the stroke volume and the cardiac output. Interobserver variability is high, mainly due to measurements of the outflow tract. Small variations in the measurement of LVOT can lead to variations in the calculation of the stroke volume of up to 20%. Nonetheless, the VTI value in the LVOT can give us an initial approximation of cardiac output assessment (normal values between 18 cam and 22cm). | ||
| C2. Pre-ejection parameters: These methods study the pre-ejection period. They are, by definition, more independent of load and more related to the intrinsic contractility of the myocardium. | ||
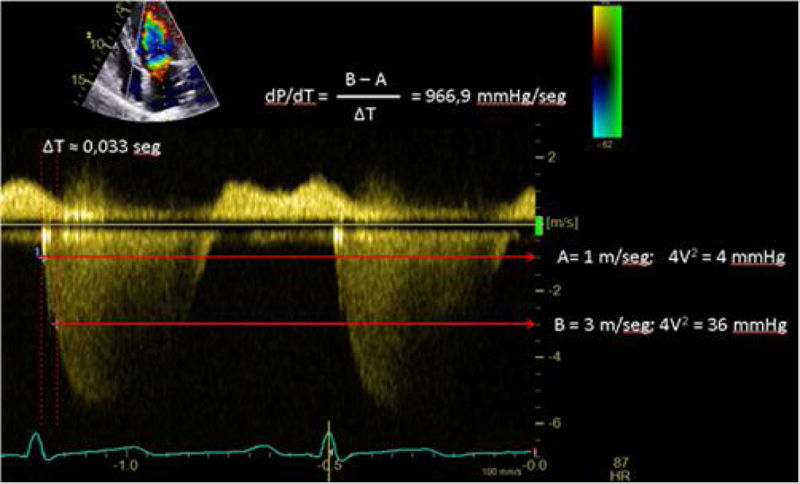
| C.2.1. dP/dt: The rate of ventricular pressure change with respect to time (dP/dt) is a parameter more widelu used during left ventricular cardiac catheterization. On the echocardiography, although we cannot measure it, in the presence of mitral regurgitation, we can estimate the ratio of the velocity of its curve (dV/dt). It should not be used in cases of acute mitral regurgitation, significant aortic stenosis, high blood pressure, or overt asynchrony, where decreased values do not imply myocardial dysfunction. | ||
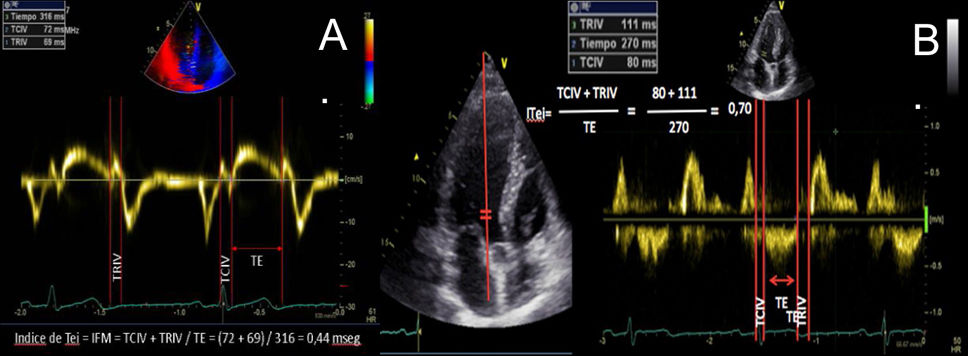
| C.2.2. Tei index or myocardial performance index (MPI): it allows for a comprehensive estimation of both ventricular (right or left) systolic and diastolic functions. It is based on the relationship between the ejection and non- ejection work of the heart. It is defined as the ratio of the sum of isovolumetric relaxation time and isovolumetric contraction time, divided by the ejection time (Tei Index=IVRT+IVCT)/ET). This prognostic index is not widely used in the routine clinical practice but tends to increase in cases of systolic and/or diastolic ventricular dysfunction. | ||
| It is a simple, reproducible method, independent of the heart rate and blood pressure, to estimate the severity and prognosis of myocardial involvement and as a predictor of postoperative left ventricular dysfunction in patients with mitral regurgitation with apparently normal ejection fraction (EF). A preoperative Tei index > 0.5 predicts postoperative LV dysfunction in patients with mitral regurgitation and apparently normal EF. We can measure it using Doppler tissue imaging (DTI) at the level of the mitral annulus (A) or the pulsed-wave spectral DTI by placing the wide sample volume between the LVOT and the mitral valve (B). The DTI can also be used to measure the Tei Index of the RV, keeping in mind that RV IVRT is virtually non-existent in the normal RV. This parameter is unaffected by heart rate or preload and has shown correlation with the severity and prognosis of various conditions affecting the RV. Its normal value according to the DTI is 0.39, and it is considered pathological when it is > 0.55. | ||
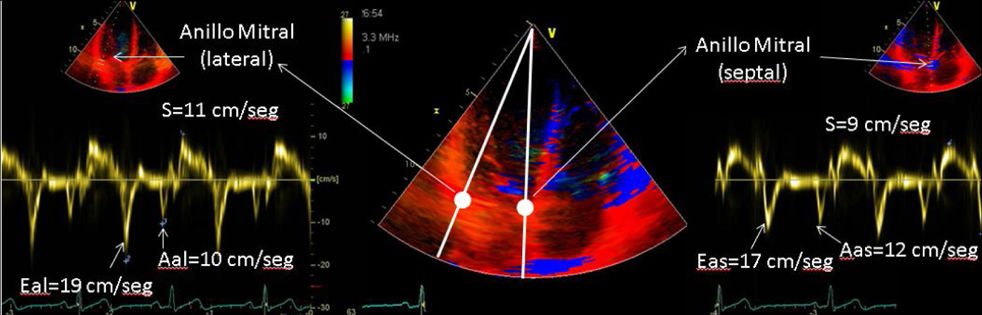
| C.3. Doppler tissue imaging (DTI): The velocity of the mitral annulus or basal myocardium measured by DTI can be a simple approach to quantify the longitudinal ventricular function and is less dependent on loading conditions. However, it is not applicable in cases associated with mitral valves, mitral annuloplasty, or severe annular calcification. An S wave with a velocity < 8cm/sec calculated by pulsed-wave spectral DTI at various sites around the mitral annulus (septal, lateral, and posterior) has a 94% sensitivity rate, a 93% specificity rate, and a 94% accuracy for the detection of EF<40%. | ||
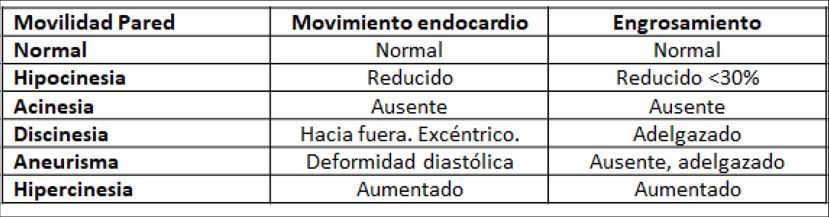
| D. | Regional systolic function | |
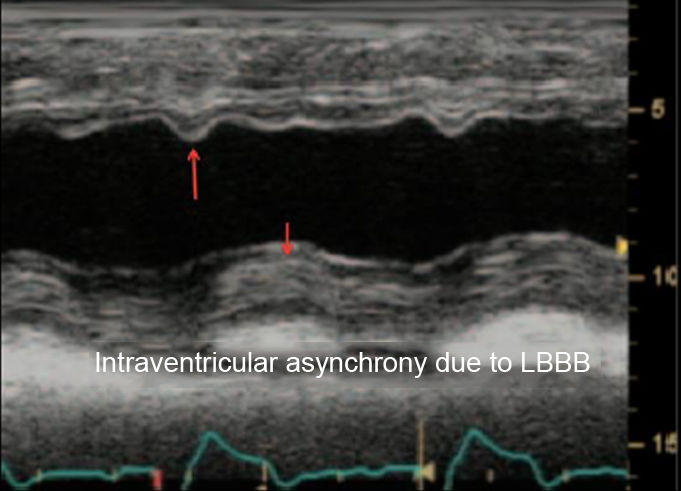
| The proper assessment of regional function requires good endocardial definition, visualization of all walls in various views, and confirmation that segmental abnormalities seen in one view are confirmed in a different view. Situations in which abnormal motility is accompanied by normal thickening (left bundle branch block, pacemaker rhythm, post-cardiac surgery, pericardial constriction) should be distinguished from ischemic changes, where abnormal motility is accompanied by changes to myocardial thickness. | ||
Regarding the assessment of right ventricular (RV) function, which is discussed in a different chapter of this series, the tricuspid annular plane systolic excursion (TAPSE) and the peak systolic velocity (PSV)7,11,12 are the most widely used parameters because they are easy to estimate and because they have a low interindividual variability. Similarly, these parameters are influenced by preload and afterload,13 and should not be used in cases of tricuspid annulus implantation or prosthesis or severe annular calcification.
It is recommended that systolic functional assessment of the RV should be performed by changing fractional area (FA), with TAPSE and PSV serving as additional parameters.14
When there are difficulties to quantitatively assess the LVEF, visual estimation is the most widely used method to study the systolic function. While this method shows an acceptable correlation with ventriculography when performed by experienced observers, its accuracy decreases with less experience and, in the case of ventricles of abnormal morphology with non-homogeneous contraction, segmental contractility abnormalities, or the presence of asynchrony.15
Ultrasound-guided assessment of hemodynamic stateEchocardiography has gained special relevance thanks to its accessibility at the bedside, non-invasiveness (in the case of TTE), and ability to provide anatomical and functional information.16
The process of hemodynamic resuscitation aims primarily at achieving adequate tissue perfusion pressure to restore proper oxygen supply to the cells. In addition to optimizing hemoglobin and blood oxygenation, it is essential to optimize the cardiac output (CO). This optimization should be guided by goals and the therapeutic response monitored to prevent or, at least, minimize the deleterious effects of these measures. Echocardiography provides relevant information about on the CO and its determinants, allowing us to assess the response to the therapeutic measures implemented.
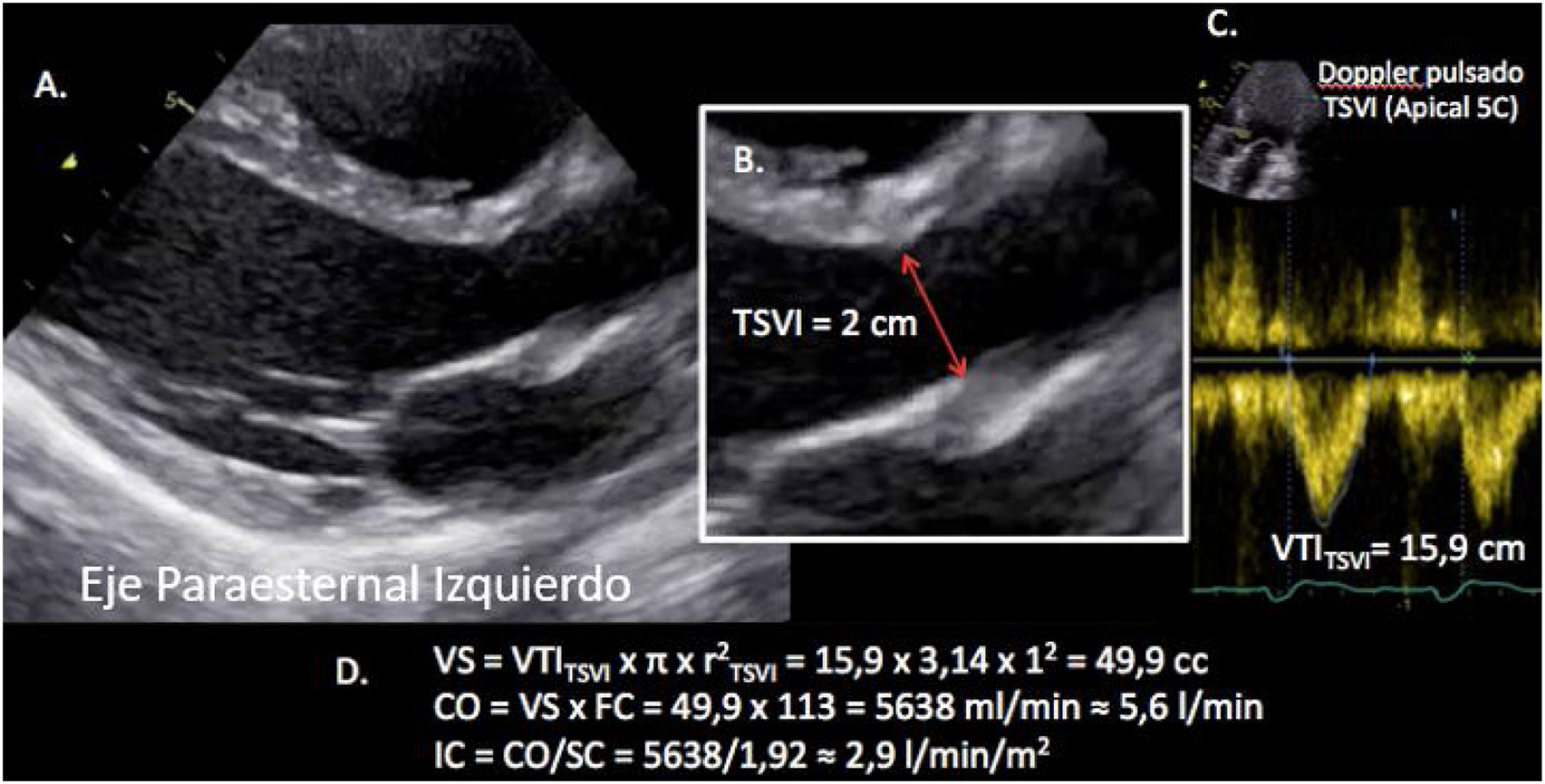
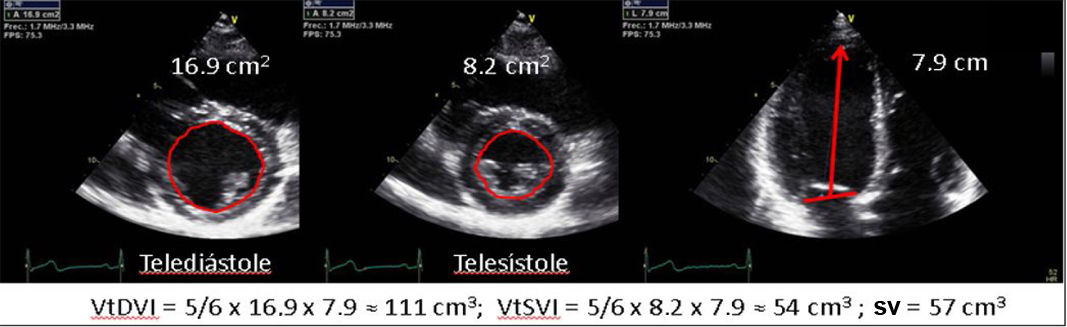
Sistolic volume (SV) assessment (Fig. 2)It can be performed using the velocity time integral (VTI) and the diameter of the left ventricular outflow tract (LVOT).
Estimation of systolic volume (SV) and cardiac output (CO) through the velocity time integral (VTI) and radius (r) of the left ventricular outflow tract (LVOT). A: left parasternal view of the left ventricle in systole. B: zoomed LVOT. C: pulsed-wave Doppler LVOT 5C apical. D: calculations.
VTI LVOT<11cm is correlated with a cardiac index<2L/min.17,18
Preload optimizationExcessive fluid administration can lead to complications, with estimates suggesting that up to 50% of the patients receiving fluids will not experience an increased SV. Therefore, before administering fluids, it is necessary to identify those patients who will benefit from them. In addition to clinical and laboratory findings of hypoperfusion indicative of the need for performing resuscitation measures, it is necessary to identify those patients who will improve their SV after fluid administration. Ultrasound, through various estimations such as the inferior vena cava collapsibility index, allows us to identify fluid-responsive patients.
As a guide for fluid administration, various pressures such as central venous pressure (CVP) or right atrial filling pressure have historically been used. Although their validity is limited by ventricular function abnormalities, such as diastolic dysfunction or postoperative myocardial stunning, extreme values can guide decision-making. An increased CVP suggests volume overload, which is associated with more complications. When assessing hepatic venous flow using pulsed-wave Doppler, a diastolic component greater than the systolic one suggests venous congestion, which, if severe, can even lead to reversed waves.19
Another variable that can be used is right atrial filling pressure, which has a good correlation with the tricuspid E/e' ratio. Values > 6 can be indicative of elevated pressures. However, there is no consensus on the utility of this parameter in the CPO.20
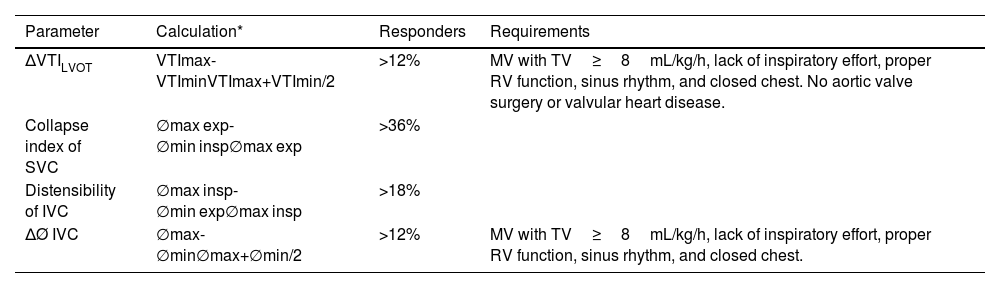
Because of the limitations described with static parameters, the current trend is to estimate dynamic variables to identify patients in preload-dependence situations. Table 26 illustrates various dynamic parameters and the requirements that must be met for their interpretation to be valid.
Dynamic echocardiographic parameters of volume responsiveness.
| Parameter | Calculation* | Responders | Requirements |
|---|---|---|---|
| ΔVTILVOT | VTImax-VTIminVTImax+VTImin/2 | >12% | MV with TV≥8mL/kg/h, lack of inspiratory effort, proper RV function, sinus rhythm, and closed chest. No aortic valve surgery or valvular heart disease. |
| Collapse index of SVC | ∅max exp-∅min insp∅max exp | >36% | |
| Distensibility of IVC | ∅max insp-∅min exp∅max insp | >18% | |
| ΔØ IVC | ∅max-∅min∅max+∅min/2 | >12% | MV with TV≥8mL/kg/h, lack of inspiratory effort, proper RV function, sinus rhythm, and closed chest. |
exp, expiration; insp, inspiration; IVC, inferior vena cava; LVOT, left ventricular outflow tract; max, maximum; min, minimum; MV, mechanical ventilation; Ø diameter; RV, right ventricle; SVC, superior vena cava; TV, tidal volume; VTI, velocity time integral.
As can be seen at the CPO, many of these determinations lose reliability when the necessary requirements for their correct interpretation are not met, such as the presence of respiratory efforts, RV dysfunction, or the occurrence of arrhythmias. In addition to the parameters described in the table, other maneuvers may be useful, such as passive leg raising, which is considered positive when there is a >10% increase in CO after it (limited estimation in the presence of increased intra-abdominal pressure or extreme hypovolemia), or the end-expiratory occlusion test, which also allows the identification of responders.
Ultrasound-guided assessment of respiratory functionAt the CPO there is a high risk of developing respiratory failure after the surgical procedure due to a reduced respiratory reserve caused by inflammatory factors following extracorporeal circulation, mechanical and hemodynamic factors.21,22
It is associated with high morbidity and mortality rates,23 making it necessary to optimize perioperative respiratory care to improve outcomes after CS.
It is well-known that the diagnostic performance of lung ultrasound exceeds that of chest x-rays in the diagnosis of lung consolidation, lung collapse, pleural effusion, pulmonary edema, and pneumothorax.24 Lung ultrasound is also highly beneficial for etiological evaluation and diaphragmatic functional assessment.25
Due to space limitations and the fact that this topic is covered in another chapter of this series, we refer the reader to chapters number 3 and 4 of this series for further information.
Assessment of the most common complications at the CPODetermining factors such as preexisting ventricular dysfunction, associated valvular heart disease, left ventricular hypertrophy, basal septal hypertrophy, pulmonary vascular disease/pulmonary hypertension (PH), or arrhythmias can not only impact accurate assessments but also the appropriate interpretation of echocardiographic findings.
Myocardial dysfunction and low postoperative COIn addition to preexisting ventricular dysfunction, myocardial function can be significantly compromised due to various factors, particularly in the presence of extended pump times. The information from the intraoperative echocardiographic study needs to be supplemented with constant assessment in the postoperative period. Echocardiographic assessment is essential as it rules out other causes of low cardiac output, such as bleeding and/or vasoplegia after Extracorporeal Circulation.
Postoperative RV dysfunction has been studied for decades, and its etiology is multifactorial. The incidence of RV dysfunction varies depending on the definition and evaluation methods used, and it can be predicted in cases of pre-existing PH or air embolism.
The decrease in traditional RV systolic function indices (TAPSE, PSV of the RV lateral wall) is well known. However, the etiology is not fully understood. One hypothesis for this phenomenon is the vulnerability of the RV to poor protection during extracorporeal circulation, geometric changes of the RV associated with the paradoxical motion of interventricular septum, or RV adhesion to the chest wall at the postoperative period, leading to a reduced RV longitudinal shortening. After pericardiotomy, the RV contractility pattern changes, resulting in a reduced longitudinal shortening and an increased transverse shortening. Of note, many echocardiographic parameters are not applicable to assess RV function after tricuspid valve surgery, and functional assessment based on FA changes is advised.26
LV dysfunction can be predictable due to preexisting dysfunction or extended periods of cardioplegia and/or extracorporeal circulation. Certain patients are especially susceptible, such as those with significant LV hypertrophy and/or coronary ischemia.
In practical terms, the trend of the LVOT systolic volume estimated by pulsed-wave Doppler is more relevant than LVEF.
Volume status assessment and dynamic gradient (video 1 of the supplementary data)Volume status assessment using ultrasound is complex and requires being integrated with clinical examination. At the CPO, certain classical volume responsiveness concepts are not applicable due to the high incidence of arrhythmias, ventricular dysfunction, PH, chronic cor pulmonale, vascular diseases, and valve replacement procedures. However, echocardiography adds value to other devices, especially to assess the left ventricular end-diastolic area, transmitral Doppler inflow and LVOT variation, and inferior vena cava collapsibility (Table 2).
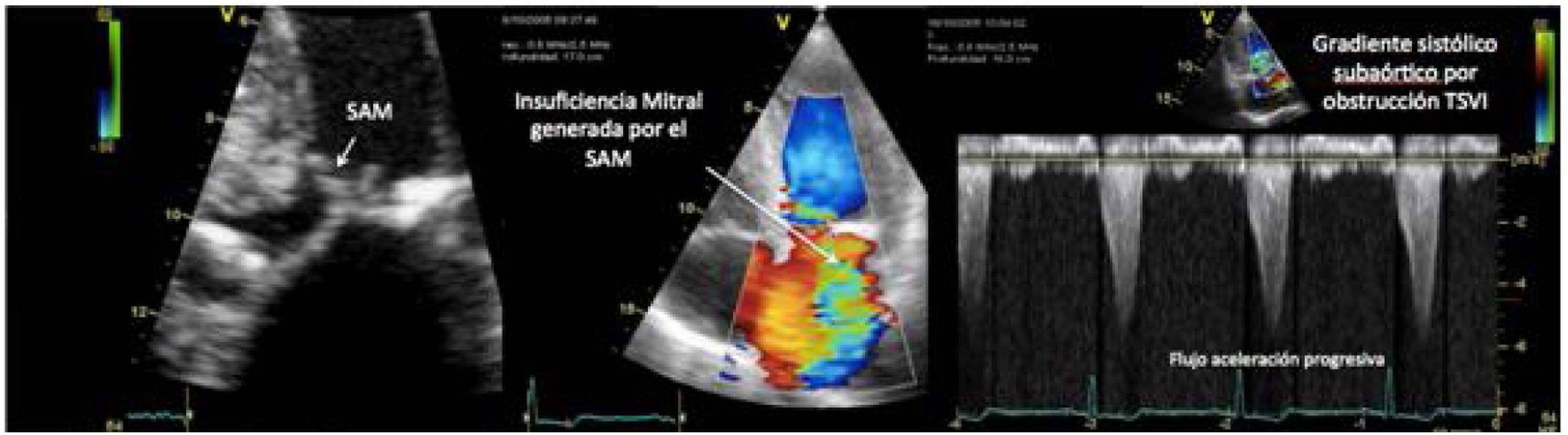
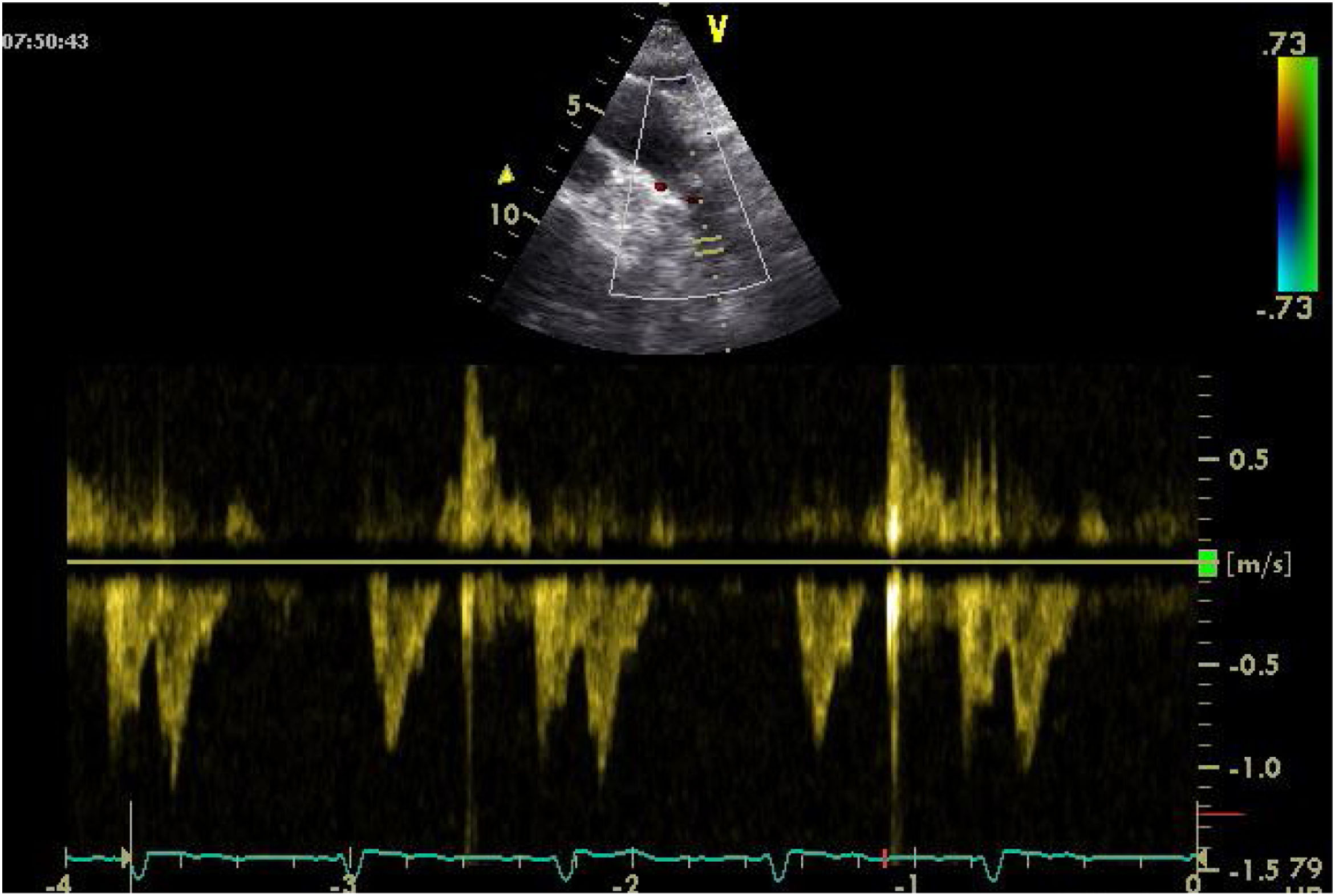
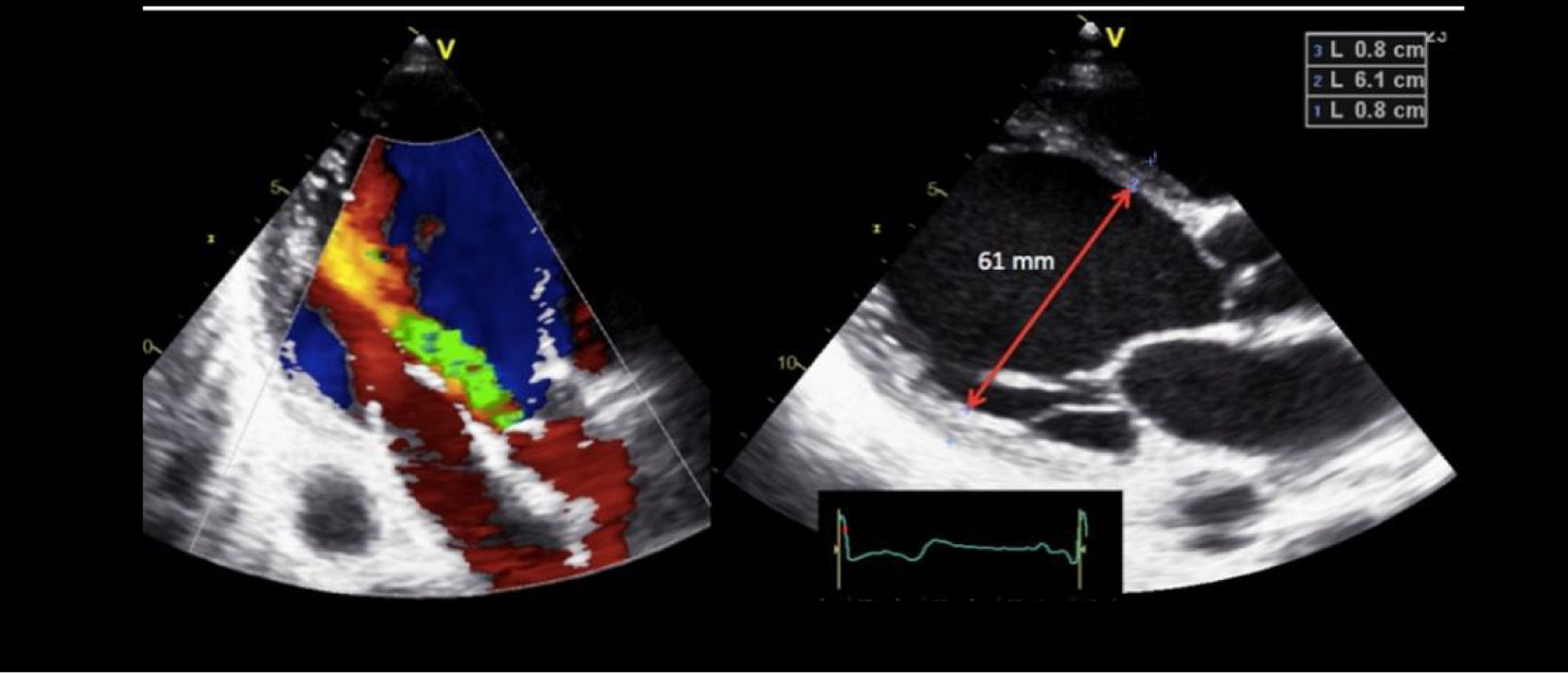
One of the most challenging clinical scenarios to manage in the CPO setting is mitral regurgitation due to abnormal systolic anterior motion (SAM), generating a pressure gradient in the LVOT or mesoventricular region, dragging and sucking the mitral anterior leaflet (Fig. 3). This should be suspected in cases with concentric hypertrophy, myectomy, or large post implantation toward the LVOT, states of hypovolemia, and/or need for ascending vasoactive support without an appropriate tensional response.
Coronary artery bypass graft (CABG) in a patient with concentric LV hypertrophy: dynamic obstruction of LVOT due to excessive inotropic support and postoperative hypovolemia, leading to severe mitral regurgitation due to anterior septal motion (SAM). Note the progressive acceleration flow on the continuous-wave Doppler.
Echocardiography is highly useful to assess the size of pericardial effusion (PE) and the degree of hemodynamic compromise. Sometimes, a simple transthoracic 4-chamber view is enough to assess PE, along with a subxiphoid long-axis view if dressings allow it.
Risk factors are advanced age, the need for emergent surgery in patients with prior anticoagulant or antiplatelet therapy, aortic root surgery, complex surgery, or poorly controlled anticoagulation in the perioperative period.
The diagnosis of cardiac tamponade is clinical and totally challenging in the CPO setting. Because the pericardium is open with drains in both the retrosternal and retropericardial spaces, it complicates its diagnosis with transthoracic echocardiography significantly. In the immediate CPO, PEs tend to be loculated, asymmetric, and located posteriorly in the atrioventricular groove and RV lateral wall. PEs are often associated with factors that change classic clinical and echocardiographic findings of tamponade, such as mechanical ventilatory support, PH, or pre-existing RV dysfunction.
In these circumstances, waiting for the onset of classic clinical and echocardiographic signs of tamponade often leads to delayed surgeries or inappropriate therapies.
In contrast, PEs that occur 72h after cardiac surgery are often larger and exhibit classical signs of cardiac tamponade.
The posterior location of PEs may require transesophageal studies to be able to see small loculated effusions causing atrial collapse (mid-esophageal 4- and 2-chamber views).
Prosthetic dysfunction (video 3 of the supplementary data)In a preliminary assessment using TTE we should pay special attention to the appearance, opening and closing of valve leaflets or occluders, the presence of leaflet calcifications, abnormal echogenic density adhered to the annulus suture, the occluder, the leaflets, the stents, or the cage. Also, we should be aware of any abnormal rocking motion occurring during the cardiac cycle. Completing the assessment with TEE is required for thorough evaluations.
- •
The thickening of the aortic root due to the presence o hematoma or edema after valve implantation can be mistaken for the presence of perianular abscesses.27
- •
The rocking motion of a valve is a sign of aortic position dehiscence. For the mitral position, when the entire native valve has been removed, an increased motion of a normal valve can be seen. The differential diagnosis will be based on the presence of a regurgitant jet.28
- •
The appearance of paravalvular leak is always pathological,29,30 being more common in valve reinterventions, elderly patients, annular calcium debridement, and aortic or mitral reconstructions. The percentage of annular circumference damaged determines the severity of paravalvular leak and can be a sign of endocarditis in a later stage.
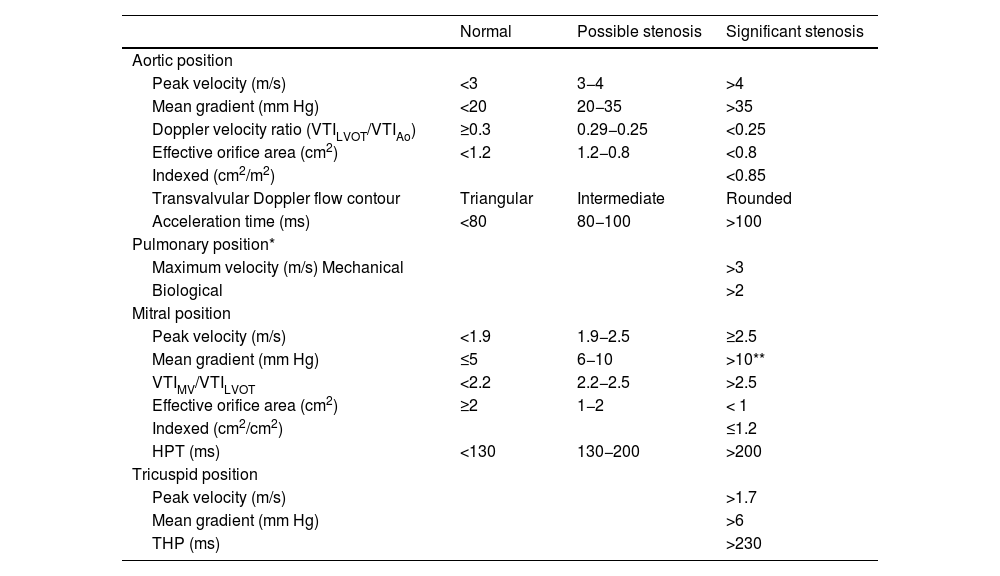
All prosthetic valves create a certain degree of obstruction compared to native valves depending on their design, size, and annular position (intra/supra), resembling stenosis. It is advisable to consult expected gradients for the diameter and model of the prosthesis (appendices 1 and 2).31–35 Therefore, it can be difficult to differentiate hemodynamic obstruction from a mild pathological dysfunction and prosthesis-patient mismatch (PPM) or mismatch (Table 3, Fig. 4, video 4 of the supplementary data).36
- •
Elevated gradients can be observed in normally functioning small-sized valves, in hyperdynamic states, or increased SV, post-valvular pressure recovery phenomena, PPM, and stenosis.
- •
In the presence of LV dysfunction, low gradients can be found with significant stenosis.
- •
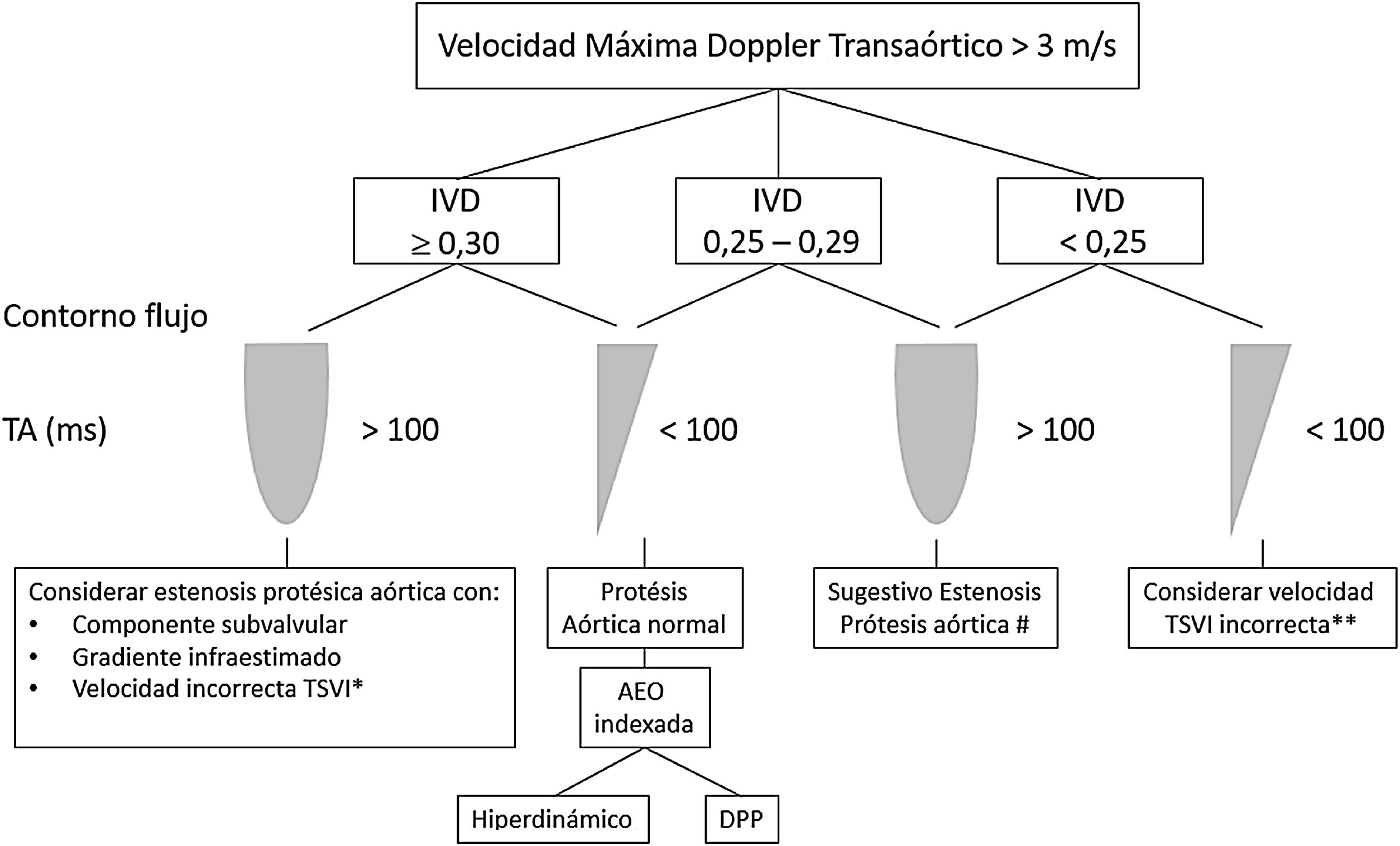
In the aortic position, the triangular morphology of the Doppler velocity contour with a short acceleration time provides us with information of normal functioning. As obstruction progresses, the Doppler velocity contour will become more rounded, until it reaches a delayed peak flow (Fig. 4).
Figure 4.* Pulsed-wave Doppler sample volume very close to the prosthesis. # Stenosis additionally confirmed by the EOA compared with reference values if valve type and size are known. ** Pulsed-wave Doppler sample volume distant (apical) from the prosthesis. AT, acceleration time; DLVOT, diameter of the LVOT; DVI, Doppler velocity index or nondimensional functional index; EOA, effective orifice area; indexed EOA, EOA divided by body surface area; LVOT, left ventricular outflow tract; PPM, prosthesis-patient mismatch; VTI, velocity-time integral.
Modified from Zoghbi WA, et al.
- •
In the mitral position, along with an elevated mean gradient, an elongated hemi-pressure time will be present.
- •
In addition to valvular gradients, it is of paramount importance to obtain flow-independent parameters of the prosthetic valve function, such as the effective orifice area (EOA) and the Doppler velocity index (DVI) or the dimensionless index (DI) (Table 3). The DVI value obtained in the early postoperative phase can serve as a control value or “valve footprint” for future studies. As long as the prosthetic valve function is normal, the DVI will remain constant even in the presence of SV changes.
- •
The combined assessment of the parameters described will establish the degree of prosthetic obstruction (Table 3).37
Doppler parameters of aortic, pulmonary, mitral, and tricuspid valve prosthetic function.
| Normal | Possible stenosis | Significant stenosis | |
|---|---|---|---|
| Aortic position | |||
| Peak velocity (m/s) | <3 | 3−4 | >4 |
| Mean gradient (mm Hg) | <20 | 20−35 | >35 |
| Doppler velocity ratio (VTILVOT/VTIAo) | ≥0.3 | 0.29−0.25 | <0.25 |
| Effective orifice area (cm2) | <1.2 | 1.2−0.8 | <0.8 |
| Indexed (cm2/m2) | <0.85 | ||
| Transvalvular Doppler flow contour | Triangular | Intermediate | Rounded |
| Acceleration time (ms) | <80 | 80−100 | >100 |
| Pulmonary position* | |||
| Maximum velocity (m/s) Mechanical | >3 | ||
| Biological | >2 | ||
| Mitral position | |||
| Peak velocity (m/s) | <1.9 | 1.9−2.5 | ≥2.5 |
| Mean gradient (mm Hg) | ≤5 | 6−10 | >10** |
| VTIMV/VTILVOT | <2.2 | 2.2−2.5 | >2.5 |
| Effective orifice area (cm2) | ≥2 | 1−2 | < 1 |
| Indexed (cm2/cm2) | ≤1.2 | ||
| HPT (ms) | <130 | 130−200 | >200 |
| Tricuspid position | |||
| Peak velocity (m/s) | >1.7 | ||
| Mean gradient (mm Hg) | >6 | ||
| THP (ms) | >230 | ||
HPT, hemi-pressure time; VTIAo, velocity time integral of the aortic valve (continuous-wave Doppler); VTILVOT, velocity time integral of left ventricular outflow tract (pulsed-wave Doppler); VTIMV, velocity time integral of the mitral valve (continuous-wave Doppler).
DE, the TEE in particular, initially provides us with the necessary information for decision-making (the need for a device and the type or types of devices that should be implanted), and the identification of conditions that may contraindicate or complicate implantation (aortic valve disease, presence of a patent foramen ovale, thrombosis, dissection, arterial vascular disease, hypertrophic cardiomyopathy, etc.).
Once the type of device that should be implanted has been selected, the insertion process, exact positioning of the cannulas, detection of complications, assessment of the effectiveness of the device implanted, and the weaning process are extremely helpful.
Intra-aortic balloon pump (IABP) (Fig. 5)- •
Check the presence of the guidewire in the descending aorta, and make sure that the probe is 2−5cm away from the left subclavian artery. If the subclavian can’t be spotted, the aortic arch lower border can be used as a reference.
- •
It's important to visualize the guidewires throughout the entire process. Transesophageal bicaval and modified bicaval views provide us with a proper view of the inferior vena cava, superior vena cava, tricuspid valve, and right atrium.38
- •
The proper position of the cannula can be determined by TTE or TEE when needed (video 5 of the supplementary data).
- •
It allows us to determine the cause of inadequate flows that happen to be a common phenomenon during ECMO support.
- •
TEE can be used to see the guidewire for the cannula return in the upper abdominal aorta and prevent inadequate placement of the cannula in any vascular branch.39 The optimal location of the venous drainage cannula through the femoral vein is in the right atrium.
- •
Monitoring the diameter of the heart chambers with TTE/TEE is essential to ensure proper emptying of the ventricles and the opening of the aortic valve. Increased afterload caused by peripheral VA-ECMO, along with severe LV dysfunction, can lead to aortic valve closure and potential clot formation in intracardiac chambers.
- •
Monitoring biventricular function allows the early detection of improvement that should be assessed by modulating ECMO flows. If there is recovery, ventricular contractility will be increased, or will be maintained without RV dilation and without an impaired stroke volume (SV) assessed with the VTI at LVOT level.
Ultrasound plays an important role in the pre-implantation evaluation, during implantation, and at the follow-up.
Previous considerations:
- •
The possibility of a right-to-left shunt through a patent foramen ovale requires exclusion before implantation and correction if detected.
- •
Similarly, moderate or severe aortic regurgitation, severe mitral stenosis, and the presence of intracavitary thrombi should be ruled out.
- •
Tricuspid regurgitation (TR) may predispose to RV failure after left ventricular assist device implantation.
After left ventricular assist device implantation, TTE/TEE will allow us to assess:
- •
Right ventricular function: RV failure after left ventricular assist device implantation occurs in up to 20%–25% of the cases and may require right ventricular assist device implantation in 10%–15% of the cases.
- •
Malfunction due to device thrombosis: In cases of evident thrombosis, the TTE is often enough for diagnosis. However, in very few cases there is direct evidence of thrombus formation. Suspicion should be based on the presence of LV dilatation, increased mitral regurgitation, and the opening frequency of the aortic valve.
In a normally functioning ventricular assist device, as the revolutions per minute increase, the left ventricular diameter, aortic valve opening, and mitral regurgitation decrease as the transmitral deceleration time increases. When the opposite thing happens, device thrombosis should be suspected.
- •
Malfunction due to suction: Any situation that decreases preload can lead to suction effects. The ventricular assist device creates decompression in the dilated LV, creating negative suction at the inflow cannula located at the cardiac apex. This can cause septal movement towards the left. A reduced preload can result in insufficient filling of the assisted ventricle, causing normal cardiac structures to approach the cannula positioned in the cardiac apex, potentially leading to cannula obstruction due to suction of adjacent cardiac structures (trabeculae, papillary muscles, septum). This can lead to reduced CO and the development of arrhythmias.40
- •
Weaning: Assessment of myocardial recovery is based on hemodynamic and echocardiographic criteria (LVEF>40%–45% with and end-diastolic diameter<45mm–60mm).
- •
Echovascular assessment of the iliofemoral axis prior to percutaneous placement.
- •
Evaluation of proper positioning of the Impella catheter. This should be performed whenever position alarms are triggered, when flows are lower than expected, or when signs of hemolysis are observed (Table 4).
Table 4.Evaluation of Impella catheter placement.
• Catheter inlet area 3.5cm below the aortic valve annulus. • Catheter outlet zone well above the aortic valve. • Catheter angled towards the apex of the LV, away from the heart wall, without kinking or blocking the mitral valve. • Dense color Doppler mosaic pattern of turbulence above the aortic valve near the catheter outlet area. - •
Assessment of preload and ventricular function.
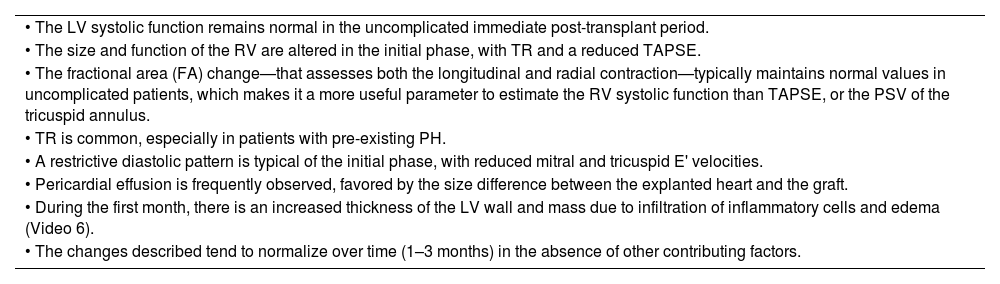
Orthotopic heart transplant is the most common type of transplant, and the surgical technique can be either biatrial or bicaval. For echocardiographic evaluation purposes, the donor heart should be normal size, and the space left by the recipient's heart should be larger, tending to rotate clockwise and locate more medially in the mediastinum. Table 5 illustrates the general considerations in the evaluation of the transplanted heart44 (video 7 of the supplementary data).
General considerations in the evaluation of the transplanted heart in the immediate postoperative period.
| • The LV systolic function remains normal in the uncomplicated immediate post-transplant period. |
| • The size and function of the RV are altered in the initial phase, with TR and a reduced TAPSE. |
| • The fractional area (FA) change—that assesses both the longitudinal and radial contraction—typically maintains normal values in uncomplicated patients, which makes it a more useful parameter to estimate the RV systolic function than TAPSE, or the PSV of the tricuspid annulus. |
| • TR is common, especially in patients with pre-existing PH. |
| • A restrictive diastolic pattern is typical of the initial phase, with reduced mitral and tricuspid E' velocities. |
| • Pericardial effusion is frequently observed, favored by the size difference between the explanted heart and the graft. |
| • During the first month, there is an increased thickness of the LV wall and mass due to infiltration of inflammatory cells and edema (Video 6). |
| • The changes described tend to normalize over time (1–3 months) in the absence of other contributing factors. |
Taking this into consideration, echocardiography helps us rule out the most common causes of hemodynamic instability that can occur in any post-cardiac surgery patient, as described earlier. Once these have been ruled out, we should focus on the more specific complications of heart transplantation:
Primary graft dysfunction- •
Early graft failure is the leading cause of death within the first 30 days after heart transplantation.
- •
It should be suspected in the presence of biventricular or RV dysfunction (damage to the LV is less common), accompanied by low CO, hypotension, and elevated filling pressures within the first 24h after transplantation.
Echocardiography helps with the early detection of RV failure, the optimization of therapeutic measures to reduce RV afterload, and the functional assessmen at the follow-up.
- •
RV failure is a well-known complication after heart transplantation, present in 50% of cardiac complications and 19% of early deaths.45
- •
Given the high frequency of RV dysfunction and associated morbidity, protection should be initiated in the operating room and continued in the postoperative period for several days, regardless of whether dysfunction is evident.
- •
Pretransplant PH in heart transplant recipients increases the risk of post-transplant PH and EV dysfunction in the donor heart.
- •
Postoperative stenosis in the pulmonary artery anastomosis can contribute to or be the main cause of RV failure.
It can occur silently or in a hyperacute form. A normal LVEF does not rule out acute rejection. TTE is an additional or alternative technique for monitoring asymptomatic rejection or clinical suspicion of rejection.
It should be suspected if the following parameters are detected:
- •
Decreased LVEF, increased RV systolic volumes, and reduced RV systolic function.
- •
Persistent restrictive diastolic patterns along with decreased mitral and tricuspid E' velocities.
None declared.
Conflicts of interestNone declared.
The following are Supplementary data to this article:
Most common femoro-jugular configuration: 1. Position of the femoral drainage cannula at intrahepatic inferior vena cava level or next to its junction with the right atrium. 2. The distance between the drainage and return cannulas should be somewhere between 7 cmm and 10cm (prevents recirculation).
Please cite this article as: Carrillo López A, Llanos Jorge C, Jiménez Rivera JJ, Clau-Terre F. Ecografía en el postoperatorio de cirugía cardíaca. Med Intensiva. 2023. https://doi.org/10.1016/j.medin.2023.08.005