To assess mortality and different clinical factors derived from the development of atraumatic pneumothorax (PNX) and/or pneumomediastinum (PNMD) in critically ill patients as a consequence of COVID-19-associated lung weakness (CALW).
DesignSystematic review with meta-analysis.
SettingIntensive Care Unit (ICU).
ParticipantsOriginal research evaluating patients, with or without the need for protective invasive mechanical ventilation (IMV), with a diagnosis of COVID-19, who developed atraumatic PNX or PNMD on admission or during hospital stay.
InterventionsData of interest were obtained from each article and analyzed and assessed by the Newcastle-Ottawa Scale. The risk of the variables of interest was assessed with data derived from studies including patients who developed atraumatic PNX or PNMD.
Main variables of interestMortality, mean ICU stay and mean PaO2/FiO2 at diagnosis.
ResultsInformation was collected from 12 longitudinal studies. Data from a total of 4901 patients were included in the meta-analysis. A total of 1629 patients had an episode of atraumatic PNX and 253 patients had an episode of atraumatic PNMD. Despite the finding of significantly strong associations, the great heterogeneity between studies implies that the interpretation of results should be made with caution.
ConclusionsMortality among COVID-19 patients was higher in those who developed atraumatic PNX and/or PNMD compared to those who did not. The mean PaO2/FiO2 index was lower in patients who developed atraumatic PNX and/or PNMD. We propose grouping these cases under the term COVID-19-associated lung weakness (CALW).
Evaluar la mortalidad y diversos factores clínicos derivados del desarrollo de neumotórax (NTX) y/o neumomediastino (NMD) atraumáticos en pacientes críticos como consecuencia de la debilidad pulmonar asociada a COVID-19 (DPAC).
DiseñoRevisión sistemática con metaanálisis.
ÁmbitoUnidad de Cuidados Intensivos (UCI).
ParticipantesInvestigaciones originales en las que se evaluase a pacientes, con o sin necesidad de ventilación mecánica invasiva (VMI), con diagnóstico de COVID-19 que hubiesen desarrollado NTX o NMD atraumáticos al ingreso o durante su estancia hospitalaria.
IntervencionesSe obtuvieron los datos de interés de cada artículo que fueron analizados y evaluados por la Escala Newcastle-Ottawa. El riesgo de las variables de interés principales se evaluó por los datos derivados de los estudios que incluyeron a pacientes que desarrollaron NTX o NMD atraumáticos.
Variables de interés principalsMortalidad, estancia media en la UCI y PaO2/FiO2 media en el momento diagnóstico.
ResultadosSe recogieron datos de 12 estudios longitudinales. En el metaanálisis se incluyeron datos de un total de 4.901 pacientes, entre los cuales 1.629 presentaron un episodio de NTX y 253 de NMD atraumáticos. A pesar de encontrar asociaciones significativamente fuertes, la alta heterogeneidad entre los estudios hace que la interpretación de los resultados deba hacerse con cautela.
ConclusionesLa mortalidad de los pacientes COVID-19 fue mayor en los que desarrollaron NTX y/o NMD atraumáticos con respecto a los que no lo hicieron. La media del índice PaO2/FiO2 fue menor en los pacientes que desarrollaron NTX y/o NMD atraumáticos. Proponemos agrupar bajo el término deDPAC estos casos.
Mechanical ventilation (MV) has become a basic resource in the Intensive Care Unit (ICU). Its use is not without risks, however.
Damage related to MV can occur de novo or it may contribute to perpetuate or worsen pre-existing thoracic injuries, with an impact on cardiopulmonary function.
Such disorders particularly include acute respiratory distress syndrome (ARDS).
The latter is characterized by an important decrease in lung compliance, and this predisposes the patient to an increased risk of barotrauma even when using MV with protective parameters.
One of the main developments in recent decades in the field of MV has been improved knowledge and control of the complications associated with the use of the technique.1–3
During the COVID-19 pandemic, there has been an increase in the number of cases of atraumatic pneumothorax (PNX) and pneumomediastinum (PNMD) in patients infected with SARS-CoV-2, with incidences that exceed those expected in the ICU setting.4
It is difficult to know whether this increase in incidence is really due to complications of SARS-CoV-2 infection, increased diagnostic performance of the ordered tests, or to other factors related to excessive care burden in the ICU (management of MV by less experienced professionals, a greater number of patients assigned to each physician, etc.).
Some studies suggest that SARS-CoV-2 infection intrinsically increases the risk of spontaneous PNX.5,6
This respiratory virus could be responsible for the lung weakness these patients develop and which exposes them to the risk of PNX and/or PNMD - both spontaneous and related to barotrauma - even when using noninvasive MV (NIMV) or MV with protective ventilation parameters.
Thus, the objective of this review was to evaluate mortality and different clinical factors related to the development of PNX, PNMD and/or pneumopericardium (PNPC), both of an atraumatic nature and possibly associated with barotrauma (patients subjected to NIMV or invasive MV [IMV]) in critical patients as a consequence of COVID-19-associated lung weakness (CALW).
MethodsLiterature searchA systematic review was made of the available scientific literature, based on the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.7
The study protocol was registered and published in the International Prospective Register of Systematic Reviews” (PROSPERO): Ref. CRD42022359045.
A search was made of the Medline, Web of Science Core Collection (Thomson Reuters) and Scopus (Elsevier) databases, covering the period up to 1 August 2022.
The search strategy was carried out by combining the appropriate MeSH terms and Boolean operators such as ((Pneumothorax) AND (COVID-19)) OR ((pneumomediastinum) AND (COVID-19)) OR ((barotrauma [MeSH terms]) AND (SARS COV-2[MeSH terms])).
In addition, we examined the references of the included articles in order to increase the sensitivity of the search.
Study selection criteriaThe search was limited to original research, including observational studies (cohort designs or case-control studies) published in English or Spanish, and to open access articles.
Other types of articles were excluded, such as case reports, research notes or communications at congresses.
As eligibility criteria, the selected studies were required to involve adult patients with a laboratory test diagnosis of COVID-19 based on reverse transcription polymerase chain reaction (RT-PCR), and with clinical conditions in the form of atraumatic PNX, PNMD or PNPC either at initial presentation or manifesting during the management of SARS-CoV-2 pneumonia.
Accordingly, and based on the PICO question, the study population (P) comprised patients with COVID-19, the intervention (I) was the clinical presentation of PNX and/or PNMD, the comparison (C) was the absence of the clinical presentation of PNX and/or PNMD, and the outcome (O) was mortality - with the study design (S) being longitudinal trials.8
Primary outcome and quality assessmentThe primary outcome of interest was mortality stratified according to the occurring event. The secondary outcomes of interest were the triggering risk factors for PNX and/or PNMD and the impact of the latter upon the duration of the ICU stay.
Quality assessment of the included studies was based on the application of the 9-star Newcastle-Ottawa Scale (NOS)9 by the first three authors independently.
The risk of bias referred to selection, compatibility and outcomes was evaluated.
High quality (low risk of bias) corresponded to studies with 8–9 stars, medium quality (medium risk of bias) to studies with 6–7 stars, and low quality (high risk of bias) to studies with ≤5 stars.
Data extraction and synthesisData extraction from the selected studies was carried out by the first three authors independently, in order to guarantee abidance with the PRISMA guidelines.
A predefined standard form was used to collect the information of interest from the selected studies.
The following data were entered in the form:
Name of the first author.
Type of study design.
Year of publication.
Country in which the study was made.
Age of the study population.
Gender.
Associated comorbidities at the time of diagnosis.
Type of event: PNX, PNMD or PNPC.
Type of ventilatory support: none, conventional low-flow oxygen therapy, high-flow nasal oxygen (HFNO), NIMV or IMV.
Gas exchange parameters such as PaO2/FiO2.
Clinical impact assessed by in-hospital mortality.
Quantitative variables were reported as odds ratios (ORs) and differences of means (DMs), with 95% confidence intervals (95%CIs).
Use was made of the inverse-variance weighting method with a random effects model, and heterogeneity was analyzed based on the I2 statistic with conventional cut-off points for high (>75%), moderate (50%–75%) and low heterogeneity (<50%), along with the Cochran Q-test, adopting a level of significance of P<.20.
Two-tailed tests were performed, with statistical significance being accepted for P<.05.
The Cochrane Review Manager 3.0 package was used throughout.
In addition, the Egger test was used to analyze the sensitivity of risk of bias, based on the Stata V.15 statistical package (Stata Corp., College Station, TX,
USA).
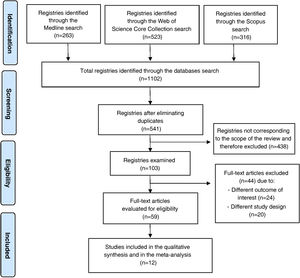
ResultsIncluded studies and flowchartThe initial search yielded a total of 1102 published articles.
The identification and exclusion of duplicates reduced the total to 541 articles for screening.
After the screening and eligibility assessment process, only 12 articles were seen to meet the inclusion criteria for our review.10–21
The article selection process is summarized in Fig. 1.
Flowchart of the selection of the studies included in the systematic review according to the PRISMA guidelines.7
The 12 included studies contributed data corresponding to a total of 4901 patients.
The geographical distribution of the studies was heterogeneous: 4 (33.33%) studies were carried out in America (exclusively in the United States), 3 (25%) studies in Europe, 4 (33.33%) in the Middle East, and 1 (8.33%) in Asia.
Most of the included patients were males: 62.34% of the total (n=3060).
The most frequent comorbidities at the time of patient admission were diabetes mellitus (72.27% of the total), arterial hypertension (68.18%), chronic obstructive pulmonary disease (COPD) (59.09%), chronic kidney disease (45.45%), coronary disease (45.45%) and cancer (31.81%).
All the studies included the appearance of PNX in the study population, except two articles.20,21
The development of PNMD was recorded in 9 of the included studies (75%), and PNPC in two (16.66%).
Table 1 shows the main characteristics of the selected studies.
Principal characteristics of the selected studies.
| Study and year | Country | N | Agea | Gender: n (%) | Comorbidities: n (%) | Ventilatory support: n (%) | Event | ICU staya |
|---|---|---|---|---|---|---|---|---|
| Cohort studies | ||||||||
| Taha et al. 2022 | USA | 334 | 61±14 | M:188 (56%) | COPD:75 (22); Asma:34 (10); AHT:266 (79); DM:200 (60); CD 67 (20); CHF:68 (20); Ictus:49 (14); CKD:56 (16); AD:18 (5) | HFNO: 7 (2.1); NIMV: 28 (8.4); IMV: 299 (89.5) | PNX | 23 (3–100) |
| F: 148 (44%) | ||||||||
| Paul et al. 2021 | India | 45 | 53.3±14.9 | M: 37 (82.2%) | DM: 17 (65.3); AHT: 11 (42.3); CD: 5 (19.2); Hypothyroidism: 2 (7.6); Cancer: 5 (19.2); CKD: 2 (7.6); Hypertriglyceridemia: 1 (3.8); COPD/Asthma: 1 (3.8) | HFNO: 8 (17.8); NIMV: 10 (22.2); IMV: 7 (15.5); O2 conventional: 16 (35.6); none: 4 (8.9) | PNX. PNMD | NA |
| F: 8 (17.7%) | ||||||||
| Özdemir et al. 2021 | Turkey | 427 | 59.9±16.1 | M: 288 (67.4%) | NA | NIMV: 73 (17.1); IMV: 354 (82.1) | PNX. PNMD | NA |
| F: 139 (32.6%) | ||||||||
| Gazivoda et al 2021 | USA | 281 | NA | M: 192 (68.3%) | Arrhythmia: 26 (9.3); Asthma: 22 (7.8); CD: 48 (17.1); COPD: 17 (6); DM: 147 (52.3); Hyperlipidemia: 102 (36.3); AHT: 169 (60.1) | IMV: 281 (100) | PNX | 15 (5−22.5) |
| F: 89 (31.7%) | ||||||||
| Guven et al. 2021 | Turkey | 75 | 60±17.9 | M: 51 (68%) | CD. AHT. DM | IMV: 75 (100) | PNX | 36.0±16.4 |
| F: 24(32%) | ||||||||
| Belletti et al. 2021 | Italy | 116 | NA | M: 98 (84.5%) | Arrhythmia: 7 (6); Stroke: 6 (5.2); AHT: 52 (44.8); Asthma: 4 (3.5); COPD: 2 (1.7); Chronic neurological problem: 3 (2.6); CKD: 7 (6); DM: 19 (16.4); Cancer: 4 (3.5) | IMV: 116 (100) | PNX. PNMD | 28 (14.5−51) |
| F: 18 (15.5%) | ||||||||
| Ozsoy et al. 2021 | Turkey | 70 | 59.15±13.85 | M: 32 (45.7%) | AHT: 37 (52.9); DM: 29 (41.4); Asthma: 17 (24.3); COPD: 2 (2.9); CKD: 7 (10); Cancer: 10 (14.3); CD: 5 (7.1) | No MV: 56 (80); MV: 14 (20) | PNX. PNMD | NA |
| F: 38 (54.3%) | ||||||||
| Elsaaran et al. 2021 | Kuwait | 343 | NA | M: 70 (65.4%) | NA | NIMV: 64 (18.8); IMV: 279 (81.2) | PNX. PNMD. PNPC | 14.9±7.8 |
| F: 37 (34.6%) | ||||||||
| Jones et al. 2020 | United Kingdom | 83 | NA | M: 61 (73.5%) | Asthma: 10 (12.8); Connective tissue disease: 4 (4.8) | NIMV: 34 (41); IMV: 49 (59) | PNX. PNMD | NA |
| F: 22 (26.5%) | ||||||||
| Kahn et al. 2021 | USA | 75 | NA | M: 55 (73.3%) | AHT: 36 (48); DM II: 43 (57.3); COPD: 1 (1.3); Asthma: 2 (2.7); CKD: 14 (18.7); Cirrhosis: 3 (4); Cancer: 4 (5.3) | No IMV: 36 (48); IMV: 39 (52) | PNX. PNMD. PNPC | 17 (15−30.5) |
| F: 20 (26.7%) | ||||||||
| Lemmers et al. 2020 | Italy | 332 | NA | M: 239 (72%) | DM: 73 (22); AHT: 186 (56); COPD: 26 (7.8) | IMV: 332 (100) | PNMD | 9 (5–18) |
| F: 93 (28%) | ||||||||
| Case-control study | ||||||||
| Reis et al. 2022 | USA | 174 | 61±14 | M: 112 (64.4%) | COPD: 14 (8); CKD: 26 (14.9); Asthma: 27 (15.5); AHT:107 (61.5); CHF: 11 (6.3); DM: 78 (44.8); Obesity: 70 (40.2) | NA | PNMD | NA |
| F: 62 (35.6%) | ||||||||
N: number of subjects; NA: information not available; ICU: Intensive Care Unit; M: males; F: females; AHT: arterial hypertension; DM: diabetes mellitus; CD: coronary disease; CHF: chronic heart failure; CKD: chronic kidney disease; AD: autoimmune disease; COPD: chronic obstructive pulmonary disease; ILD: interstitial lung disease; HFNO: high-flow nasal oxygen; NIMV: noninvasive mechanical ventilation; IMV: invasive mechanical ventilation; MV: mechanical ventilation; PNX: pneumothorax; PNMD: pneumomediastinum; PNPC: pneumopericardium.
The quantitative analysis was divided into various subgroups.
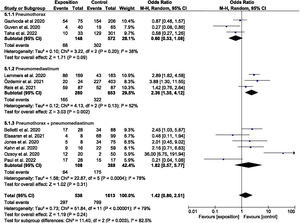
The odds ratios for the studies that evaluated mortality in atraumatic PNX, PNMD and PNX+PNMD are reported in Fig. 2.
The pooled analysis yielded OR=1.42 (95%CI: 0.80–2.51), with high heterogeneity (I2=79.00%; P<.00001), though there were differences between the evaluated subgroups.
The subgroup evaluating mortality in PNMD yielded OR=2.36 (95%CI: 1.35–4.12), though the heterogeneity index I2 was found to be 52%, evidencing statistically significant heterogeneity (P=.13).
On the other hand, mortality was evaluated and stratified according to the geographical setting of the studies (Europe+United States versus others), as can be seen in Supplementary Figure 1.
The OR was 1.51 (95%CI: 0.92–2.49) for Europe+the United States, while the OR was 1.42 (95%CI: 0.22–7.33) for the rest of countries.
Nevertheless, it should be noted that high heterogeneity persisted (I2=79.00%; P<.00001).
The difference of means for PaO2/FiO2 was −43.16mmHg (95%CI: −56.40 to −29.92) with a low heterogeneity index (I2=26%; P=.25).
These results indicate that the mean for the group of patients that developed atraumatic PNX and/or PNMD was lower than in the group that did not develop the studied event.
Specifically, the difference was greater in the subgroup that developed PNMD, with an estimated difference of means of −45.89 (95%CI: −70.45 to −21.33), but with a high heterogeneity index (I2=52%; P=.12).
However, in the case of the development of PNX, the estimated differences of means were similar to those of the previous subgroup, but with a low heterogeneity index (I2=0%; P=.68), as can be seen in Fig. 3.
It is important to point out that the limited sample size and number of studies included in the forest plot make it necessary to interpret these articles with caution.
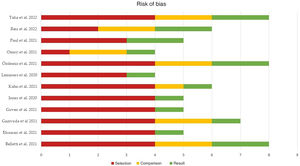
Publication and risk of bias assessmentThe quality of the studies included in the meta-analysis was assessed according to the risk of selection, comparison and outcome bias, as indicated by the NOS.
The mean score of the global studies was 5.91 (median 5.5, range 4–8).
Fifty percent of the studies (n=6) presented a high risk of bias (score≤5), one-quarter (n=3) presented a moderate risk of bias (score 6–7), and the remaining 25% (n=3) showed a low risk of bias (score 8–9).
Fig. 4 summarizes the main results of the risk of bias assessment, which can be consulted more in detail in Supplementary Figure 2.
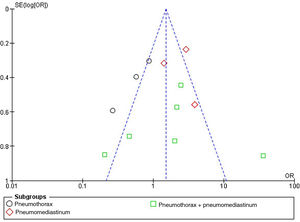
The publication bias referred to mortality risk in patients with atraumatic PNX and/or PNMD was represented by a funnel plot, as can be seen in Fig. 5. The plot evidences no significant bias.
This was verified by the Egger test for the effect of small studies, which was not statistically significant, as can be seen in Supplementary Figure 3.
DiscussionThis meta-analysis documents and combines the available information on the association of developing atraumatic PNX, PNMD or PNPC, whether spontaneous in patients not subjected to MV or attributable to barotrauma in patients subjected to MV, in some groups of patients with COVID-19 admitted to the ICU.
Although our results appear to indicate a positive association with in-hospital mortality, the summarizing estimates must be interpreted with caution due to the great heterogeneity observed and the many studies with low to medium methodological quality.
COVID-19-associated lung weakness: a disorder to be defined?The previously reported coronavirus epidemics (SARS in China, in 2002) evidenced an association between the clinical presentation of pneumonia and the development of PNX and PNMD.22
Some viruses of the SARS family are characterized by a cell penetration mechanism through angiotensin-converting enzyme II (ACE II).23
Pneumocytes have abundant ACE II receptors but are not the only cells in the human body to have such receptors.
In effect, the gastrointestinal tract is also characterized by abundant ACE II receptors, and different therapeutic strategies targeted to these molecules have been investigated in certain disease processes such as type 2 diabetes or Crohn's disease.24 Likewise, there have been reports of spontaneous pneumoperitoneum in seriously ill patients with COVID-19.6
The host response to the virus is based on the prolonged release of proinflammatory cytokines and antibodies targeted to this intracellular microorganism, and the alveolar wall is damaged as a consequence of this immune attack.
The intervention of the perialveolar macrophages accounts for the observed radiological condensation and the impossibility of adequate gas exchange, which triggers a decrease in PaO2/FiO2 and the development of ARDS.25
Such alveolar rupture has been suggested to represent the pathophysiological basis of spontaneous PNX and PNMD in non-ventilated patients.
In contrast, PNX associated with barotrauma occurs as a result of the so-called Macklin effect.
This effect accounts for the dissecting action exerted by the intrapulmonary air upon the bronchopulmonary interstice towards the lung hilum, at the mediastinal level.26
Both clinical conditions constitute medical emergencies requiring rapid intervention.
Such events are mainly managed by inserting a chest drain combined with ventilatory support involving a positive end-expiratory pressure (PEEP) as low as possible.27
These authors also consider ventilator-induced lung injury (VILI) to be a possible causal mechanism, and have proposed some preventive measures to palliate its development, such as the limitation of tidal volume and breathing effort, the optimum adjustment of PEEP to each patient, and efforts to identify which patients genuinely intubation and MV.
On the other hand, it must be mentioned that computed tomography (CT) has been crucial for diagnosis in most of the studies found in the literature.28,29
The uncertainty as to whether such conditions truly constitute a complication inherent to the disease or instead reflect the improved diagnostic performance of the ordered tests led Knox et al.5 to carry out a multicenter study evaluating the pre-pandemic cases of PNX and PNMD versus those occurring during the pandemic, in a large cohort of critically ill patients.
Their data indicated that there is indeed a greater risk of developing atraumatic PNX as a complication of COVID-19. These results are consistent with our findings, though with a lesser risk and a more limited confidence interval.
It has been demonstrated that COVID-19 causes lung weakness that may be associated with the development of atraumatic PNX and PNMD.30 The identification of such events as being atraumatic could increase the homogeneity of the studies focused on the same type of event.
For this reason, we propose the identification of a new clinical entity referred to as COVID-19-Associated Lung Weakness (CALW).
Limitations and applicability of the resultsOur study has several limitations.
Firstly, the great heterogeneity of the included studies implies that the results obtained must be interpreted with caution.
This heterogeneity is explained by the variability of the study designs and the low to medium methodological quality of most of the articles.
Secondly, mention must be made of the high risk of bias referred to adequate monitoring of the case-control study in assessing mortality in the meta-analysis.
Although the inclusion of this study increased the sensitivity of the search and minimized possible publication bias, it could complicate the interpretation of this analysis.21
On the other hand, and from a methodological perspective, no authors of previous studies were contacted to allow more in-depth knowledge of different aspects of the published data and about studies not yet published, since no articles from the grey literature were included.
Furthermore, the search strategy might not have reached optimum sensitivity and specificity levels, since the cluster search for other identified articles yielded several studies that we had not identified in the literature search.
As strong points, the present study establishes the need to identify CALW as a clinical entity, since it could improve the homogeneity of future studies and allow greater consensus in management of the critical patient.
Likewise, it could establish differences between the identification of confounding factors and factors that may truly intervene or predict the development of the events described in this study.
Lastly, the general sequelae of COVID-19 have been clinically relevant.31,32
This would make it interesting to know the possible long-term sequelae among patients who have recovered from these events.
ConclusionsMortality among COVID-19 patients was higher among those who developed atraumatic PNX and/or PNMD than in those who did not.
The mean PaO2/FiO2 was lower among the COVID-19 patients who developed atraumatic PNX and/or PNMD.
We propose the application of the term COVID-19-Associated Lung Weakness (CALW) to those cases of atraumatic PNX and PNMD in COVID-19 patients not subjected to ventilation or who receive low-pressure ventilation (tidal volume≤6−8ml/kg body weight).
Author contributionsStudy conception and design: P.R.G., M.R.I. and A.C.C.; methodology: P.R.G., A.J.L.R.B., A.C.C., A.R.L. and M.R.I.; formal analysis and data compilation: P.R.G., M.R.P. and C.J.G.; writing of the manuscript: P.R.G., M.R.P., C.J.G., P.J.G. and A.J.L.R.B.; review and editing of the manuscript, P.R.G., M.R.P., C.J.G., P.J.G., A.J.L.R.B., A.C.C., A.R.L., M.R.I. and A.C.C.; supervision, A.C.C., M.R.I. and A.J.L.R.B. All the authors have read and approved the manuscript version submitted for publication.
FundingThis study has not received funding of any kind. Financiación de la tasa de acceso abierto: Universidad de Granada/CBUA.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks are due to the Department of Medicine of the University of Granada for promoting and stimulating the introduction to clinical research among its students.