To analyze the impact of positive end-expiratory pressure (PEEP) changes on intracranial pressure (ICP) dynamics in patients with acute brain injury (ABI).
DesignObservational, prospective and multicenter study (PEEP-PIC study).
SettingSeventeen intensive care units in Spain.
PatientsNeurocritically ill patients who underwent invasive neuromonitorization from November 2017 to June 2018.
InterventionsBaseline ventilatory, hemodynamic and neuromonitoring variables were collected immediately before PEEP changes and during the following 30 min.
Main variables of interestPEEP and ICP changes.
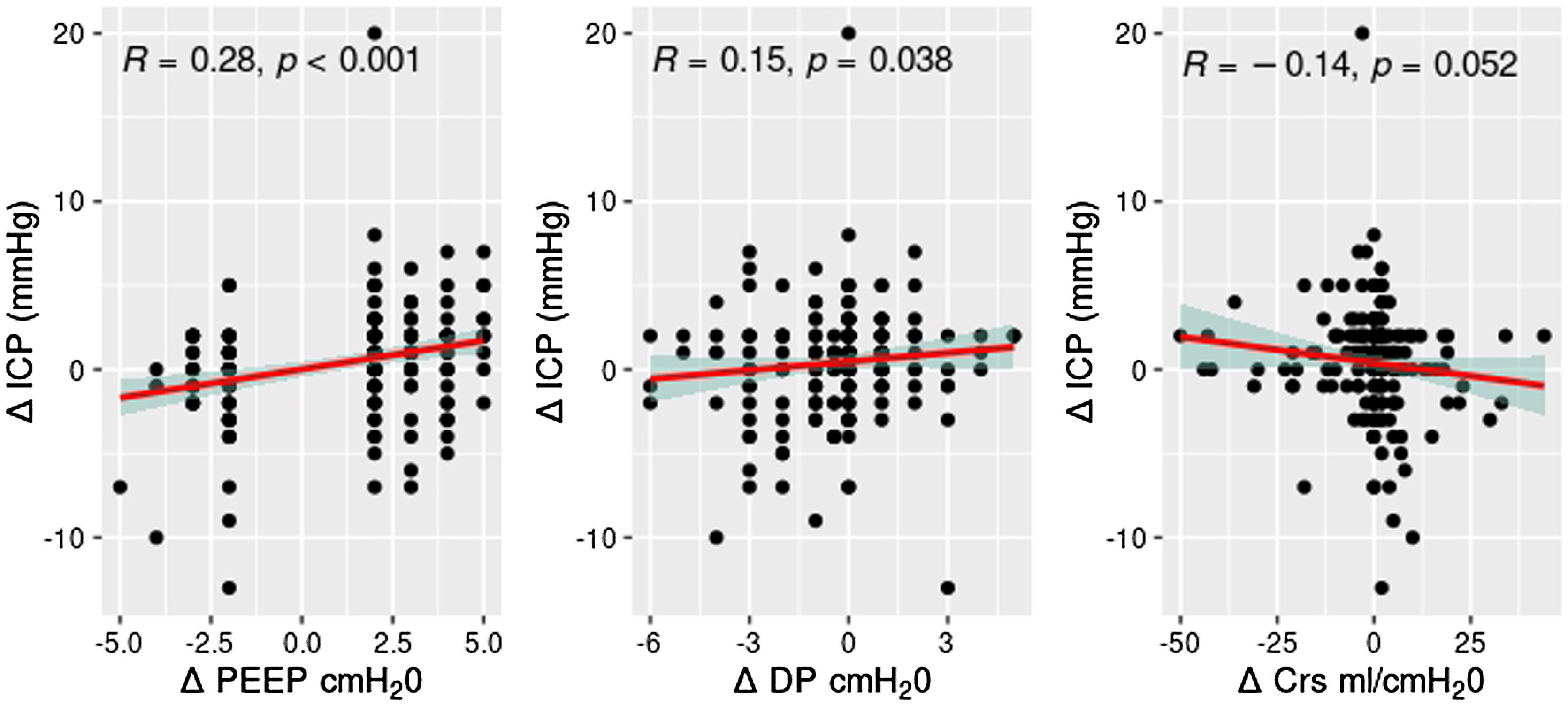
ResultsOne-hundred and nine patients were included. Mean age was 52.68 (15.34) years, male 71 (65.13%). Traumatic brain injury was the cause of ABI in 54 (49.54%) patients. Length of mechanical ventilation was 16.52 (9.23) days. In-hospital mortality was 21.1%. PEEP increases (mean 6.24–9.10 cmH2O) resulted in ICP increase from 10.4 to 11.39 mmHg, P < .001, without changes in cerebral perfusion pressure (CPP) (P = .548). PEEP decreases (mean 8.96 to 6.53 cmH2O) resulted in ICP decrease from 10.5 to 9.62 mmHg (P = .052), without changes in CPP (P = .762). Significant correlations were established between the increase of ICP and the delta PEEP (R = 0.28, P < .001), delta driving pressure (R = 0.15, P = .038) and delta compliance (R = −0.14, P = .052). ICP increment was higher in patients with lower baseline ICP.
ConclusionsPEEP changes were not associated with clinically relevant modifications in ICP values in ABI patients. The magnitude of the change in ICP after PEEP increase was correlated with the delta of PEEP, the delta driving pressure and the delta compliance.
Analizar el impacto de los cambios en la Presión positiva al final de la espiración (PEEP) sobre la Presión Intracraneal (PIC) en pacientes con lesión cerebral aguda (ABI).
DiseñoEstudio prospectivo, observacional y multicéntrico (PEEP-PIC).
ÁmbitoDiecisiete Unidades de Cuidados Intensivos en España.
PacientesPacientes neurocríticos que recibieron monitorización invasiva de la PIC desde Noviembre 2017 hasta Junio 2018.
IntervencionesSe recogieron los parámetros ventilatorios, hemodinámicos y variables de neuromonitorización inmediatamente antes de las modificaciones de la PEEP y durante los 30 minutos posteriores.
Variables de interés principalesPEEP y cambios en la PIC.
ResultadosSe incluyeron 109 pacientes. Edad media 52,68 (15,34) años, hombres 71 (65,13%). La causa de lesión cerebral fue traumática en 54 pacientes (49,54%). La estancia media fue de 16,52 (9,23) días. La mortalidad hospitalaria fue del 21,1%. Los aumentos de PEEP (media 6,24 a 9,10 cmH2O) resultaron en un aumento de la PIC de 10,4 a 11,39 mmHg, P < .001, sin cambios en la PPC (P = ,548). Los descensos de la PEEP (media 8,96 a 6,53 cmH2O) resultaron en un descenso de la PIC de 10,5 a 9,62 mmHg (P = ,052), sin cambios en la PPC (P = ,762). Se establecieron correlaciones significativas entre el aumento de PIC y el delta de PEEP (R = 0,28, P < ,001), el delta driving pressure (R = 0,15, P = ,038) y el delta de complianza (R = −0,14, P = ,052). El aumento de PIC fue mayor en pacientes con PIC basal baja.
ConclusionesLos cambios en la PEEP no se asociaron a cambios clínicamente relevantes en la PIC de los pacientes con lesion cerebral aguda. La magnitud del aumento de la PIC tras el aumento de PEEP se correlacionó con el delta PEEP, delta driving pressure y el delta complianza.
The communication pathway between the brain and the lung is called the brain-lung crosstalk.1,2 Patients with acute brain injury (ABI) have increased susceptibility to pulmonary complications through sympathetic activation, inflammation, intracranial hypertension and immune suppression.1,2 Furthermore, patients with ABI are at high risk of altered airway patency because of the neurological implication of the central nervous system (CNS) control of cough and swallowing.1 Consequently, ABI patients present lung complications like acute respiratory distress syndrome (ARDS), ventilator-associated pneumonia (VAP), and neurogenic pulmonary edema (NPE) from long-term mechanical ventilation. In turn, mechanical ventilation is not free of harmful effects to the brain due to complex pathophysiological interactions between intrathoracic, central venous and intracranial compartments.1–3
Positive end-expiratory pressure (PEEP) is defined as the alveolar pressure above atmospheric pressure at end-expiration. Careful selection of PEEP promotes alveolar patency throughout the respiratory cycle, improves pulmonary compliance, and enables more homogeneous distribution of the forces of mechanical ventilation.2 However, recently published guidelines state that what constitutes ideal PEEP to attenuate lung injury and avoid hyperinflation is unknown yet,4 since excessive PEEP may also reduce venous preload and cardiac output, attenuate jugular venous drainage, and promote alveolar overdistension.2–4 In ABI patients, these changes may potentially lead to intracranial hypertension and cerebral ischemia through a reduction in CPP.5,6
To this purpose, the objective of this observational, prospective and multicenter study (PEEP-PIC study) was to analyze the impact of PEEP changes on ICP dynamics in patients with acute brain injury.
MethodsThe PEEP-PIC study was an observational, prospective and multicenter study including neurocritically ill patients who underwent invasive neuromonitorization as a part of their standard management. The study was endorsed by the Neurointensive Care and Trauma Working Group of the Spanish Society of Intensive Care Medicine (SEMICYUC). Ethics Committee approval for the study was initially obtained in the coordinating centre (Hospital Universitario 12 de Octubre, Madrid: CEI: 17/221). Subsequently, all participating sites were required to have the study approved by their local ethics committee. Written informed consent was obtained from patients’ closest relatives.
Study populationPatients with ABI (traumatic or non-traumatic) admitted to the participating ICUs from November 2017 to June 2018 who underwent invasive neuromonitorization as a part of their standard treatment.
Inclusion criteria:
- a)
age ≥ 16 years.
- b)
Acute brain injury included:
- -
Traumatic brain injury (TBI).
- -
Non-traumatic brain injury (subarachnoid hemorrhage and intracerebral hemorrhage).
- -
- c)
Invasive mechanical ventilation.
- d)
Invasive neuromonitorization (ICP through intraventricular or intraparenchymal probe with/without brain tissue O2 probe).
Exclusion criteria:
- a)
Age < 16 years.
- b)
Inability to obtain written informed consent.
- c)
Life expectancy less than 24 h at investigator criteria.
- d)
If during the observation period, the patient is unstable and requires additional therapeutic interventions.
- e)
If During the observation period, the patient is breathing without mechanical ventilation.
- f)
If During the observation period, the patient needs additional changes in ventilatory parameters (in addition to PEEP changes).
Baseline clinical characteristics of the patients included were recorded. Baseline ventilatory, hemodynamic and neuromonitoring variables were collected immediately before PEEP changes and during the following 30 min. The decision to increase or decrease PEEP was taken independently by the attending intensivist at his/her best criteria. We evaluated a maximum of 3 episodes per patient (both in episodes with PEEP increase or decrease). The difference between the baseline and the value after PEEP increase was considered the delta value (ie, the delta of PEEP was the magnitude of PEEP increase).
No further interventions were performed unless additional changes in PEEP were necessary. In that case, the procedure was repeated. Additional information can be found at the study website (https://sites.google.com/view/peeppic/p%C3%A1gina-principal).
Data collection and statistical analysisAs per protocol, data were collected by local investigators in REDCap (Research Electronic Data Capture) hosted by Hospital Universitario 12 de Octubre, Madrid. REDCap is a secure web-based software platform designed to facilitate data capture for research studies.7,8 Outliers were substituted with median values. In cases where the percentage of missing values exceeded 10% for outcome variables, the missing values were imputed using the mean replacement method.
Quantitative variables are presented as either mean ± standard deviation (SD) or as median with the Interquartile Range (IQR), depending on appropriateness. Qualitative variables are expressed as counts (percentages). Categorical variables underwent analysis using either the χ2 test or Fisher's exact test, depending on the context. The normality of continuous data was assessed using the Shapiro-Wilk test. As continuous data exhibited a non-normal distribution, we used the non-parametric Wilcoxon test for evaluation. The relationship between ICP and respiratory variables was examined using Spearman's correlation coefficient. A P-value of less than .05 was considered statistically significant. All statistical analyses were conducted using RStudio (version 2023.3.1).
ResultsOne-hundred and nine patients were finally included. Of them, the cause of ABI was traumatic brain injury in 54 cases (49.54%) and aneurysmal subarachnoid hemorrhage or intracerebral hemorrhage in 55 cases (50.46%). Chest trauma was present in 25 patients (22.94%). Mean age was 56.68 (15.34) years, male 71 (65.13%), median Glasgow Coma Scale (GCS) score was 8 (IQR 7), mean Acute Physiology and Chronic Health Evaluation-II (APACHE-II) was 18.17 (7.27) and mean Sequential Organ Failure Assessment (SOFA) score was 6.63 (3.17).
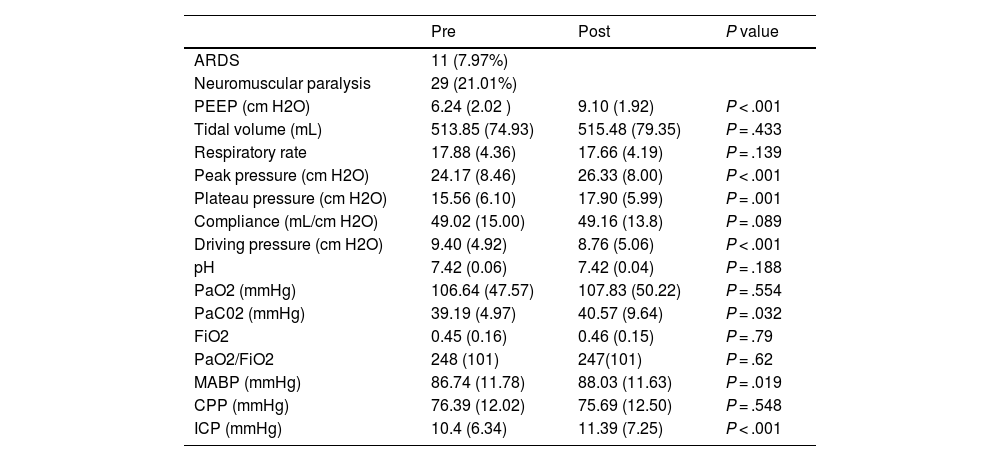
Length of mechanical ventilation of the ABI patients studied was 16.52 (9.23) days. In-hospital mortality was 21.1%. Ventilatory modes at the time the analyses were performed were: volume control (59.6%), pressure control (4.1%), pressure-regulated volume control (27.1%) and pressure support ventilation (9.2%). Ventilatory, hemodynamic and neuromonitoring responses to PEEP increase are summarized in Table 1. Ventilatory, hemodynamic and neuromonitoring responses to PEEP decrease are summarized in Table 2. Of note, the coexistence of chest trauma did not result in differences of responses of ICP (P = .276) and CPP (P = .845) responses to the same PEEP increases (P = .598) compared to patients without chest trauma. Similarly, after similar PEEP decreases (P = .962), no differences between chest trauma patients and patients without chest trauma were observed in terms of ICP (P = .621) and CPP (P = .784) responses.
Ventilatory, hemodynamic and neuromonitoring responses to PEEP increase (n = 138 episodes).
| Pre | Post | P value | |
|---|---|---|---|
| ARDS | 11 (7.97%) | ||
| Neuromuscular paralysis | 29 (21.01%) | ||
| PEEP (cm H2O) | 6.24 (2.02 ) | 9.10 (1.92) | P < .001 |
| Tidal volume (mL) | 513.85 (74.93) | 515.48 (79.35) | P = .433 |
| Respiratory rate | 17.88 (4.36) | 17.66 (4.19) | P = .139 |
| Peak pressure (cm H2O) | 24.17 (8.46) | 26.33 (8.00) | P < .001 |
| Plateau pressure (cm H2O) | 15.56 (6.10) | 17.90 (5.99) | P = .001 |
| Compliance (mL/cm H2O) | 49.02 (15.00) | 49.16 (13.8) | P = .089 |
| Driving pressure (cm H2O) | 9.40 (4.92) | 8.76 (5.06) | P < .001 |
| pH | 7.42 (0.06) | 7.42 (0.04) | P = .188 |
| PaO2 (mmHg) | 106.64 (47.57) | 107.83 (50.22) | P = .554 |
| PaC02 (mmHg) | 39.19 (4.97) | 40.57 (9.64) | P = .032 |
| FiO2 | 0.45 (0.16) | 0.46 (0.15) | P = .79 |
| PaO2/FiO2 | 248 (101) | 247(101) | P = .62 |
| MABP (mmHg) | 86.74 (11.78) | 88.03 (11.63) | P = .019 |
| CPP (mmHg) | 76.39 (12.02) | 75.69 (12.50) | P = .548 |
| ICP (mmHg) | 10.4 (6.34) | 11.39 (7.25) | P < .001 |
ARDS: Acute respiratory distress syndrome; PEEP: Positive end-expiratory pressure; PaO2: Arterial oxygen partial pressure; PaCO2: Arterial carbon dioxide partial pressure; FiO2: Inspired oxygen fraction; MABP: Mean arterial blood pressure; CPP: Cerebral perfusion pressure; ICP: Intracranial pressure.
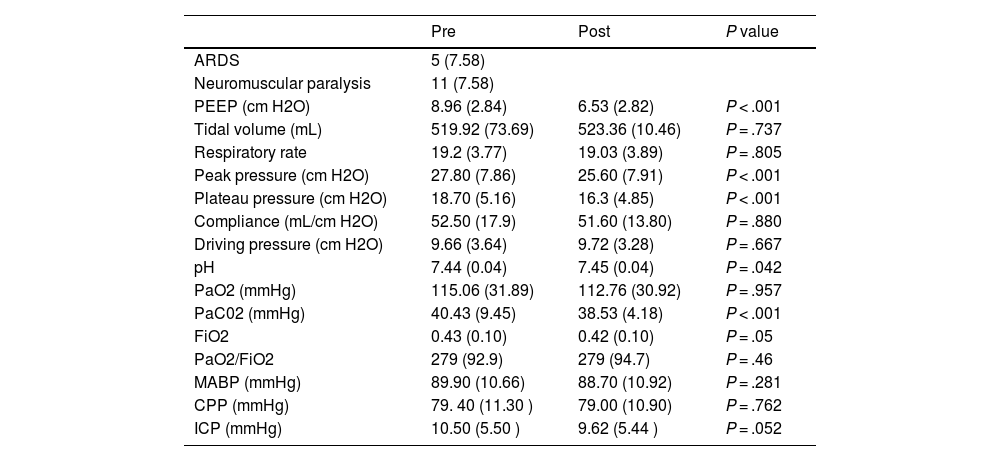
Ventilatory, hemodynamic and neuromonitoring responses to PEEP decrease (n = 66 episodes).
| Pre | Post | P value | |
|---|---|---|---|
| ARDS | 5 (7.58) | ||
| Neuromuscular paralysis | 11 (7.58) | ||
| PEEP (cm H2O) | 8.96 (2.84) | 6.53 (2.82) | P < .001 |
| Tidal volume (mL) | 519.92 (73.69) | 523.36 (10.46) | P = .737 |
| Respiratory rate | 19.2 (3.77) | 19.03 (3.89) | P = .805 |
| Peak pressure (cm H2O) | 27.80 (7.86) | 25.60 (7.91) | P < .001 |
| Plateau pressure (cm H2O) | 18.70 (5.16) | 16.3 (4.85) | P < .001 |
| Compliance (mL/cm H2O) | 52.50 (17.9) | 51.60 (13.80) | P = .880 |
| Driving pressure (cm H2O) | 9.66 (3.64) | 9.72 (3.28) | P = .667 |
| pH | 7.44 (0.04) | 7.45 (0.04) | P = .042 |
| PaO2 (mmHg) | 115.06 (31.89) | 112.76 (30.92) | P = .957 |
| PaC02 (mmHg) | 40.43 (9.45) | 38.53 (4.18) | P < .001 |
| FiO2 | 0.43 (0.10) | 0.42 (0.10) | P = .05 |
| PaO2/FiO2 | 279 (92.9) | 279 (94.7) | P = .46 |
| MABP (mmHg) | 89.90 (10.66) | 88.70 (10.92) | P = .281 |
| CPP (mmHg) | 79. 40 (11.30 ) | 79.00 (10.90) | P = .762 |
| ICP (mmHg) | 10.50 (5.50 ) | 9.62 (5.44 ) | P = .052 |
ARDS: Acute respiratory distress syndrome; PEEP: Positive end-expiratory pressure; PaO2: Arterial oxygen partial pressure; PaCO2: Arterial carbon dioxide partial pressure; FiO2: Inspired oxygen fraction; MABP: Mean arterial blood pressure; CPP: Cerebral perfusion pressure; ICP: Intracranial pressure.
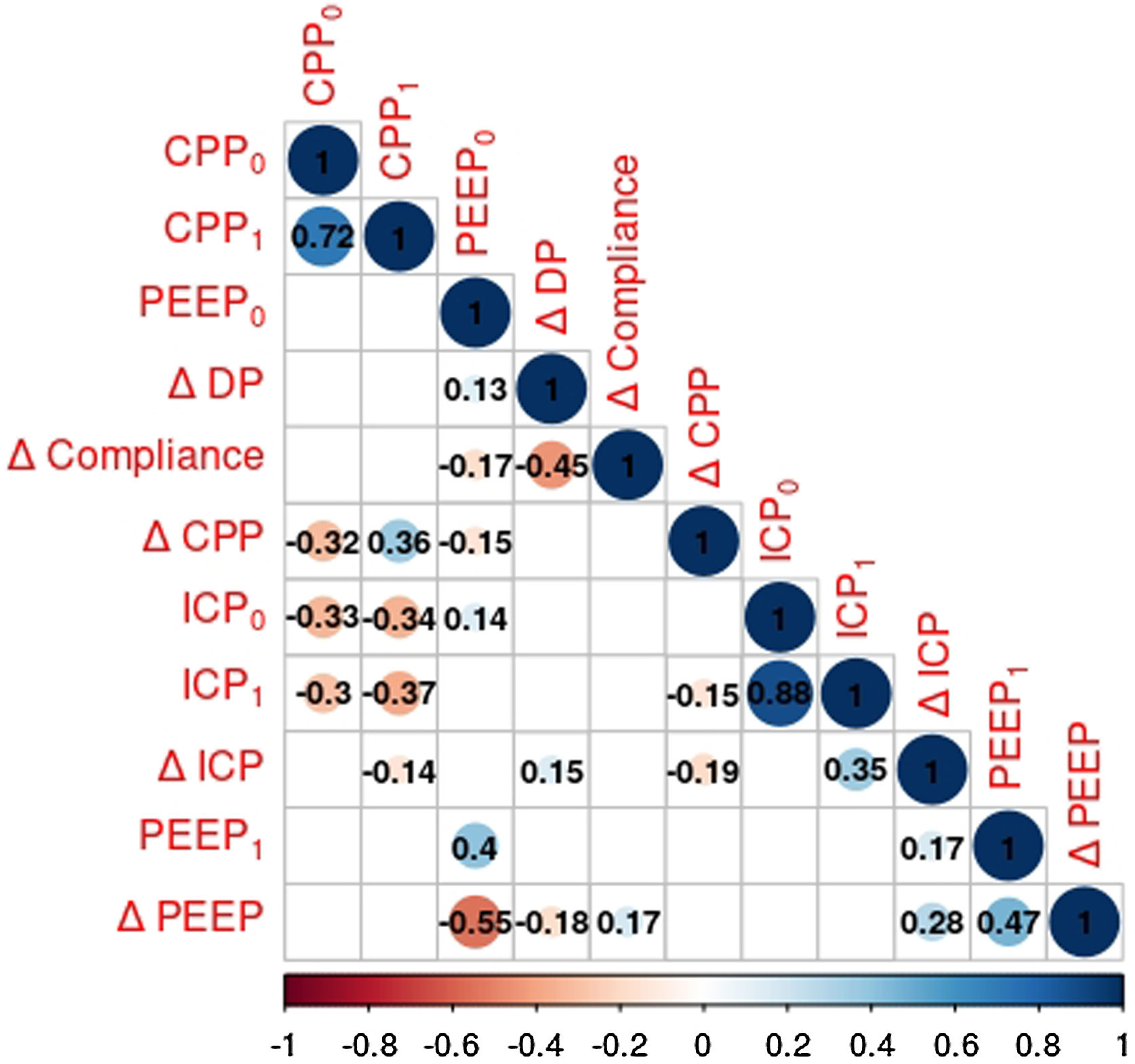
Correlations between ICP and respiratory variables using Spearman's correlation coefficient are depicted in Fig. 1.
Heat map showing the associations of ICP and respiratory variables. Pearson correlation coefficients are displayed. Circles representing the strength of correlation are omitted if they do not achieve statistical significance. Blue and red circles represent positive and negative correlations.
Significant correlations were established between the increase of ICP and the delta PEEP, delta driving pressure and delta compliance (Fig. 2).
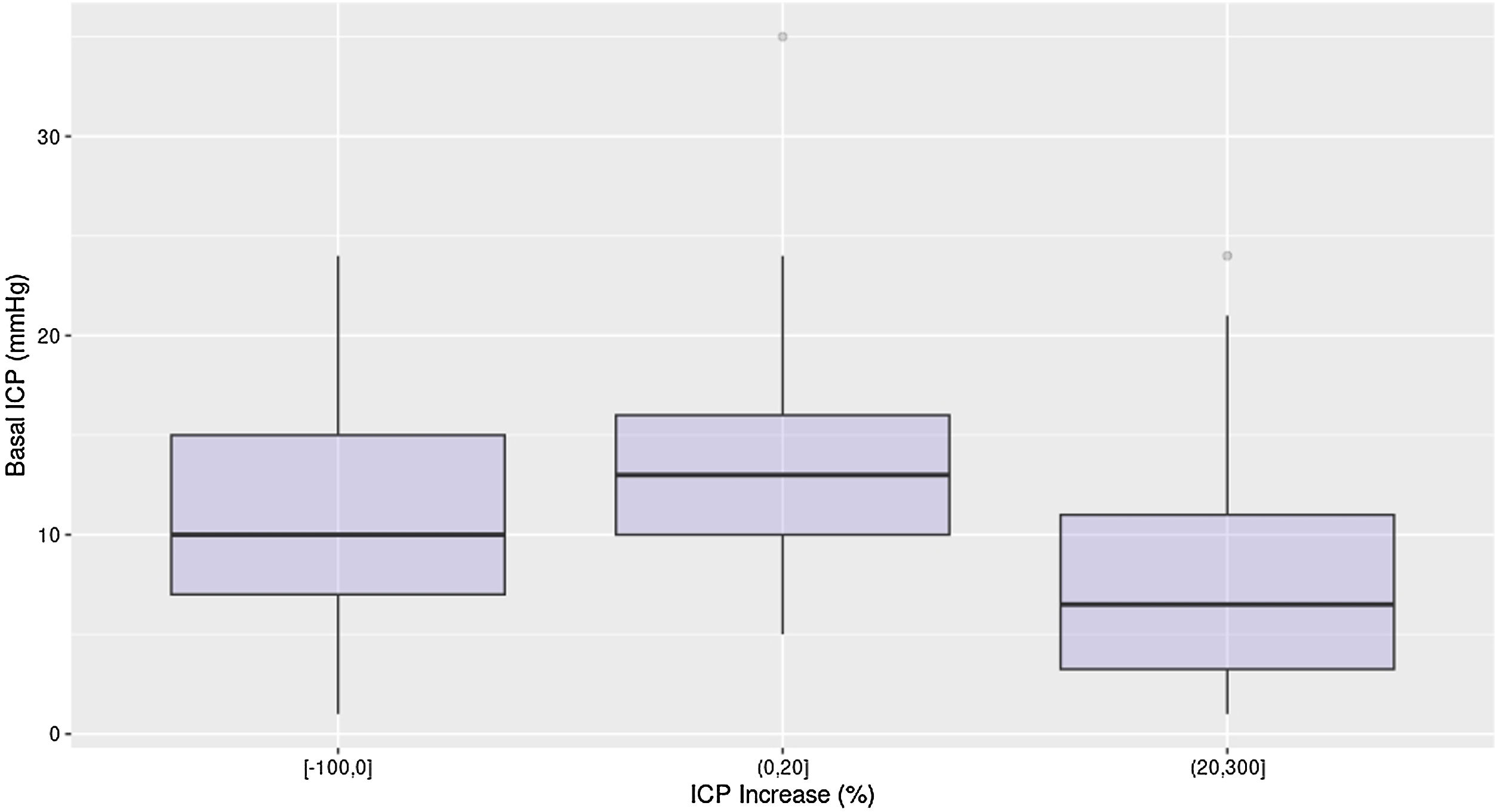
Interestingly, ICP increment was higher in patients with lower baseline ICP (Fig. 3). No correlation was found between the increase in ICP values after PEEP modifications and mortality.
DiscussionIn ABI patients, PEEP changes were not associated with clinically relevant modifications in ICP values. The magnitude of the change in ICP after PEEP increase was correlated with the delta of PEEP, the delta driving pressure and the delta compliance. Patients who had lower baseline ICP had a higher percentage of increase in ICP values.
The brain-lung crosstalk has gained attention in recent years,1,3,5,6 because different ventilatory parameters may affect cerebral dynamics. Specifically, PEEP settings might be of paramount importance, since PEEP can elevate intrathoracic and intraabdominal pressure and reduce mean arterial pressure (MAP) and cardiac output and, therefore, potentially affect ICP and cerebral perfusion pressure,1 and traditionally the trend in the ICU was maintaining low PEEP levels to avoid increases in ICP.9 We found that increments in PEEP values resulted in a slight increase in ICP without changes in CPP. However, the magnitude of this ICP increase seems not clinically relevant. Our results are in line with recent evidence suggesting that increments of PEEP can be safely applied in ABI patients10–12 without triggering additional interventions. In addition, slow PEEP augmentation did not affect cerebral oxygenation or cerebral metabolism13 and cerebral autoregulation.12,13 In this direction, Bequiri et al. have recently showed that strategies including lung protective ventilation (LPV) comprising low tidal volume (VT) and high PEEP are safe and associated with non-clinically relevant changes in ICP in most patients.14
Interestingly, in our study patients with lower baseline ICP had a higher percentage of increase in ICP values. This has been previously showed by McGuire et al. in their study including eighteen patients with ABI.15 PEEP at 5 cm H2O had no effect on ICP in the group with normal ICP, whereas PEEP at 10 and 15 cm H2O produced a significant (P < .05) increase in ICP (1.9 and 1.5 mm Hg, respectively). On the other hand, in the group with increased ICP, no significant change in ICP occurred at any of the PEEP levels used.15 In both groups, cerebral perfusion pressure remained unchanged.15 The rationale behind this finding was suggested by Li et al. In their study, they tested the hemodynamic hypothesis suggesting that PEEP might exert adverse effects on cerebral hemodynamics by impeding cerebral venous return and thereby elevating ICP. In this approach, the central venous pressure may be an intermediary that delivers pressure from PEEP to ICP, therefore leading to increases in ICP after PEEP augmentation only in patients with baseline ICP close to CVP.16
In addition, we observed that baseline respiratory parameters were not correlated with ICP increases, but the delta of PEEP and the delta driving pressure after PEEP increase were. The delta of compliance of the respiratory system was inversely correlated (in this case in the limit of statistical signification). Taken together, these data suggest that ICP increases depend mainly on the respiratory responses to ventilator settings and the intrathoracic pressures, driving to the need of an appropriate personalization of mechanical ventilation17 and appropriate intensive care management.18 The effect of PEEP on ICP may rely on several factors (lung recruitability, chest wall elastance, respiratory mechanics, intracranial compliance or baseline ICP), and the results may be unchanged, increased, or even decreased ICP according to PEEP increase.19–21 Robba et al. suggested that PEEP may have a detrimental effect on ICP only when it causes alveolar hyperinflation leading to a significant increase in PaCO2, whereas when PEEP leads to good alveolar recruitment, ICP does not change.22 If intrathoracic pressures are transmitted into the intracranial veins, it can increase the volume of intracranial blood (even if only slightly). If an incremental increase in the volume of the intracranial contents exceeds the “compensatory reserve,” ICP will rise precipitously23 even as a part of an intracranial compartmental syndrome.24 In this direction, Li et al. proposed that the intracranial-to-central venous pressure gap predicts the responsiveness of ICP to PEEP in patients with TBI16 which may be of special interest since in a recent study including ICP increases in 142/295 episodes of PEEP increments (58%), no baseline variable was able to identify this response.25
Our study has some limitations: first, although the number of patients studied was high, the number of patients with baseline high ICP was low. However, since the delta ICP was higher in patients with low baseline ICP we believe that these results can be extrapolated to the whole ABI population. Second, we studied patients with relatively small changes in PEEP values in almost all cases, so we cannot rule out potential clinically relevant increases in ICP values if higher PEEP changes were analyzed. In addition, the population studied was heterogeneous (half of them from trauma and half of them being patients with aneurysmal subarachnoid hemorrhage or intracerebral hemorrhage) and we did not evaluate differences according to different etiologies,6 which may constitute a potential area of study.
In conclusion, PEEP changes were not associated with clinically relevant modifications in ICP values in patients with ABI. The magnitude of the change in ICP after PEEP increase was correlated with the delta of PEEP, the delta driving pressure and the delta compliance. Patients who had lower baseline ICP had a higher percentage of increase in ICP values.
StatementsEthics: We confirm adherence to ethical guidelines. Ethics Committee approval for the study was initially obtained in the coordinating centre (Hospital Universitario 12 de Octubre, Madrid: CEI: 17/221). Subsequently, all participating sites were required to have the study approved by their local ethics committee. Written informed consent was obtained from patients’ closest relatives.
Conflict of interest: All authors have disclosed their potential Conflicts of Interest. All authors declare no financial disclosure.
Checklist: We confirm that we have followed “The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement” checklist.
Funding: The study was performed without external funding.
Authors contributionConceptualization: Jesús Abelardo Barea-Mendoza and Mario Chico-Fernández.
Formal analysis: Jesús Abelardo Barea-Mendoza.
Funding aquisition: N/A.
Investigation: Jesús Abelardo Barea-Mendoza, Zaira Molina Collado, María Ángeles Ballesteros-Sanz, Luisa Corral Ansa, Maite Misis del Campo, Cándido Pardo Rey, Juan Angel Tihista Jiménez, Carmen Corcobado Márquez, Juan Pedro Martín del Rincón, Juan Antonio Llompart-Pou, Luis Alfonso Marcos Prieto, Ander Olazabal Martínez, Rubén Herrán Monge, Ana María Díaz Lamas, Mario Chico-Fernández.
Methodology: Jesús Abelardo Barea-Mendoza and Mario Chico-Fernández.
Supervision: Mario Chico-Fernández.
Writing-original draft: Jesús Abelardo Barea-Mendoza and Juan Antonio Llompart-Pou.
Writing-review and editing: Jesús Abelardo Barea-Mendoza, Zaira Molina Collado, María Ángeles Ballesteros-Sanz, Luisa Corral Ansa, Maite Misis del Campo, Cándido Pardo Rey, Juan Angel Tihista Jiménez, Carmen Corcobado Márquez, Juan Pedro Martín del Rincón, Juan Antonio Llompart-Pou, Luis Alfonso Marcos Prieto, Ander Olazabal Martínez, Rubén Herrán Monge, Ana María Díaz Lamas and Mario Chico-Fernández.
The authors thank all the PEEP-PIC investigators. Special thanks to Isidro Javier Prieto del Portillo y Luis Terceros Almanza (Hospital Universitario “12 de Octubre”) for their contribution and effort in the early stages of this project.
Andrea Rodriguez Biendicho, Carlos García Fuentes (Hospital Universitario “12 de Octubre”); Javier Rodríguez Pilar (Hospital Universitari Son Espases); Íker García Sáez, Bego Azkárate Ayerdi (Hospital Universitario de Donostia); Alicia Muñoz Cantero (Hospital Universitario de Badajoz); Gerard Moreno Muñoz (Hospital Universitari Joan XXIII); Raúl de Pablo Sánchez (Hospital Universitario Ramón y Cajal); Victoria Andrea Hortigüela Martín (Hospital Fundación Jiménez Díaz); María Ángela Magro Martín (Hospital Universitario de Toledo).