To know the clinical profile as well as the prognostic significance of elevated levels of parathyroid hormone (PTH) in patients admitted for acute coronary syndrome (ACS).
Design and settingObservational and prospective study of patients admitted for ACS in a single Spanish center during a period of six months.
Intervention and variables of interestThe circulating concentrations of PTH, calcidiol, calcitriol, NT-proBNP, C-reactive protein, cystatin C and fibrinogen were determined within the first 48h at admission. We performed adjusted models to predict death or re-entry for ACS after hospital discharge.
ResultsA total of 161 patients were recruited (age 67±14 years, 75.2% were men). Forty-one (25.5%) patients had elevated PTH values. During follow-up for a period of 275 person-years, 50 adverse events were recorded. Patients with elevated PTH levels were proportionally more women (21.2 vs. 39.0%) and older (63.3 vs. 77.8 years, both p<.05). Likewise, they presented significantly more cardiovascular risk and a worse prognosis during follow-up (incidence rate ratio 2.64 CI 95%: 1.5–4.6). However, in an adjusted model by the GRACE score, PTH levels were not shown to be an independent risk factor (hazard ratio=1.1; 95% CI: 0.6–2.2), neither other components of the panel.
ConclusionsThe proportion of patients with elevated levels of PTH admitted for ACS was high. The presence of high PTH levels was associated with an unfavorable clinical profile and a worse outcome during the follow-up, although it was not an independent predictor of poor prognosis.
Conocer el perfil clínico, así como el significado pronóstico, de la presencia de niveles elevados de hormona paratiroidea (PTH) en pacientes ingresados por síndrome coronario agudo (SCA).
Diseño y ámbitoEstudio observacional y prospectivo de pacientes ingresados por SCA en un único centro español durante un periodo de 6meses.
Intervención y variables de interésSe determinaron las concentraciones de PTH, calcidiol, calcitriol, NT-proBNP, proteínaC reactiva, cistatinaC y fibrinógeno circulantes en las primeras 48h del ingreso y se realizaron modelos ajustados para predecir muerte o reingreso por SCA tras el alta.
ResultadosSe reclutaron 161 pacientes (edad 67±14años; 75,2% varones) de los cuales 41 (25,5%) presentaron valores elevados de PTH. Se registraron 50 eventos adversos durante un seguimiento de 275 personas-año. Los pacientes con niveles elevados de PTH fueron en mayor proporción mujeres (21,2 vs. 39,0%) y de mayor edad (63,3 vs. 77,8años, ambos p<0,05). Asimismo, presentaron mayor riesgo cardiovascular y una peor evolución en el seguimiento (razón de tasas de incidencia: 2,64; IC 95%: 1,5-4,6). Sin embargo, en un modelo ajustado por la escala GRACE, los niveles de PTH no se mostraron como un factor de riesgo independiente (hazard ratio=1,1; IC 95%: 0,6-2,2); tampoco el resto de componentes del panel.
ConclusionesLa proporción de pacientes con niveles elevados de PTH ingresados por SCA es elevada. Su presencia se asoció con un perfil clínico más adverso y peor evolución durante el seguimiento, aunque no resultó ser un predictor independiente de mal pronóstico.
Patients with acute coronary syndrome (ACS) constitute a very heterogeneous population, with great variability in terms of both the form of presentation of the syndrome and the risk of death or ischemia recurrence over the long term.1 The continuous search for new tools offering support for clinicians in assessing the patient prognosis is therefore essential. In addition to purely clinical variables such as cardiovascular history, comorbidities, the electrocardiogram or the presence of heart failure upon admission, biomarkers have become an essential element for diagnosis, patient stratification and management decision making.2 The Global Registry of Acute Coronary Events (GRACE) score is routinely used for the risk stratification of patients with ACS, and has been shown to be a potent tool in this respect. However, while its performance in differentiating poor outcome patients is good, it is not perfect – as suggested by its C statistic in the order of 0.8.3 This observation is of particular importance, since any improvements in the discriminating capacity of this score would be of great value in clinical practice.
Both vitamin D, in the form of calcidiol and calcitriol, and parathyroid hormone (PTH) play a key role in bone homeostasis and in the maintenance of calcium-phosphorus balance. Previous studies have demonstrated a relationship between increased cardiovascular risk and the presence of primary4 or secondary hyperparathyroidism.5 This relationship appears to be largely attributable to an increase in the prevalence of cardiovascular risk factors among patients with asymptomatic primary hyperparathyroidism.6,7 On the other hand, a number of authors have related the presence of high PTH levels to more complex coronary disease8; a suboptimum response to antiplatelet medication9; and even the presence of a greater number of adverse events during follow-up10 in patients diagnosed with stable coronary disease. However, the evidence of a potential prognostic impact of PTH in patients with ACS is practically inexistent, and is based only on a small pilot study involving 22 patients.11 No specific study designed to explore this hypothesis has been carried out to date.
The present study was therefore carried out to determine whether measurement of the circulating PTH levels offers prognostic information following the discharge of patients admitted due to ACS. We have also investigated whether the circulating levels of calcidiol and calcitriol are useful for the differentiation of patients at risk of suffering adverse events, and whether such an association persists after controlling for other more solidly established biomarkers such as the amino-terminal fraction of brain natriuretic peptide (NT-proBNP), C-reactive protein (CRP) or cystatin C.
Patients and methodsA prospective, analytical, observational cohort study was carried out with the recruitment of all patients presenting a main diagnosis of ACS admitted between 1 November 2011 and 31 April 2012 to a General University Hospital in Cartagena (Murcia, Spain; latitude 37° N). Patients not living in Spain and therefore not amenable to follow-up were excluded, as were those who failed to give informed consent to participation in the study. Data collection included: demographic information, cardiovascular risk factors, history of cardiovascular disease and chronic kidney disease (CKD), clinical and laboratory test data (albumin, hemoglobin, creatinine, cystatin C, CRP, NT-proBNP, 25-hidroxivitamina D or calcidiol [25(OH)-D], 1,25(OH) vitamin D or calcitriol [1,25(OH)-D], PTH, fibrinogen, calcium and albumin-corrected calcium) referred to admission, and events during follow-up. The study was approved by the Clinical Research Ethics Committee (CREC) of our center, and complied with the principles of the current version of the Declaration of Helsinki.
DefinitionsA history of cardiovascular disease was defined as chronic ischemic heart disease, stroke or peripheral arterial disease documented in the case history. The GRACE (version 1.0) was used as prognostic score, comprising 9 variables: age, heart rate and systolic blood pressure upon admission, creatinine concentration upon admission, the presence of elevated myocardial damage markers or ST-segment depression upon admission, a history of myocardial infarction, the presence of heart failure upon admission, and in-hospital percutaneous coronary intervention during admission.12
Laboratory methodsA venous blood sample for laboratory testing was collected at 8:00 a.m. during the first 48h of admission.
Parathyroid hormone was measured by direct chemiluminescence testing with the AdviaCentaurXP analyzer (Siemens Healthcare Diagnostic). A PTH concentration of 79.5pg/ml was regarded as the upper reference limit according to the recommendations of the manufacturer, and was used as the cut-off point for classifying patients as belonging to either the “normal PTH” or the “high PTH” group. In addition, cystatin C was assessed by immunonephelometry with the BN ProSpec analyzer (Siemens Healthcare Diagnostic); NT-proBNP was determined by chemiluminescence immunoassay based on LOCI technology using the Dimension Vista analyzer (Siemens Healthcare Diagnostic); and CRP, albumin and calcium were determined by immunoturbidimetry, bromocresol green (BCG) fixation, and the arsenazo method, respectively, using the Advia 2400 analyzer (Siemens Healthcare Diagnostic).
For the measurement of 25(OH)-D we collected a blood sample in a tube with lithium heparin, separating gel and reduced glutathione (antioxidant). Following extraction, the sample was sent in ice and protected from light to the laboratory, where it was centrifuged and the plasma fraction was separated and stored at −80°C until processing. The 25(OH)-D levels were determined by high performance liquid chromatography with ultraviolet detection using the Agilent 1200 analyzer, after a brief manual precipitation and extraction step. Radioimmunoassay (RIA) in turn was used to determine 1,25(OH)-D in serum. The glomerular filtration rate (GFR) was estimated using the formula derived from the Modification of Diet in Renal Disease study, and creatinine measurement was based on Jaffe's kinetic method compensated and standardized with respect to the reference method involving isotope dilution – mass spectrometry (IDMS) [MDRD-4 IDMS] using the Advia 2400 analyzer (Siemens Healthcare).
Statistical analysisContinuous variables were presented as the mean and standard deviation (SD), or as the median and interquartile range (IQR), as applicable, while categorical variables were presented as absolute number and percentage. The comparison of continuous variables between groups was based on the Student t-test or the Mann–Whitney U-test, as applicable, while the chi-squared test or Fisher exact test was used for the comparison of categorical variables.
Survival analysis was performed using the Kaplan–Meier graphic method and the log-rank test (Cox-Mantel). We reported the incidence rate (IR) of adverse events per 100 patients-year according to the PTH levels, together with the IR ratio with its corresponding confidence interval (CI). In addition, a Cox regression model was generated for explanatory purposes, adjusted to the GRACE score, which is regarded by the clinical guides as the gold standard for risk stratification,13 calculating the hazard ratio (HR) and the 95% confidence interval (95%CI) for each covariable in the model. The temporal variable was the time elapsed between hospital discharge and appearance of the event, or last contact with the investigator. The enter method was used, with calculation of the CI values and p-values based on the Wald statistic. In addition, the p-values were estimated by a backward stepwise method and the likelihood ratio (LR). The risk proportionality hypothesis was tested with a graphic method and also with the Schoenfeld residuals test. The principal variable (dependent variable) of the study was death due to any cause or readmission due to ACS (reinfarction or unstable angina requiring admission) after discharge. The Cox model discriminating capacity was calculated using Harrell's C-statistic. The analysis was completed with the evaluation of PTH levels in the different subgroups by studying the first-grade interactions in the hierarchical model with a backward stepwise method and the likelihood ratio (Chunk test). In addition, we investigated the diagnostic performance of PTH in predicting the combined event by calculating the area under the receiver operating characteristic (ROC) curve and using the macro !DT (Domenech JM, UAB, Barcelona, Spain). In relation to hypothesis contrast testing, an α threshold value of 0.05 was considered for rejecting/accepting the null hypothesis. The statistical analyses were carried out using the SPSS® version 23.0 statistical package (SPPS Inc., Chicago, IL, USA).
Follow-upAfter hospital discharge, all patients were followed-up on in the Cardiology clinic and/or by telephone monitoring during a median of 22 months (Q1–Q3: 8–33) for the combined event of death or ACS.
ResultsBasal characteristics of the sampleWe recruited a total of 161 patients with an age of 67±14 years. The majority (75.2%) were males. A history of arterial hypertension was recorded in 65.8%, dyslipidemia in 55.9%, and diabetes mellitus in 35.4%. In turn, 36.6% were active smokers, and 35.4% were obese. A history of cardiovascular disease was established in 47.8% of the cases, with ischemic heart disease being the most prevalent presentation (being recorded in 39.8% of the sample). In relation to admission, the diagnosis was ACS without ST-segment elevation in 62.7% of the cases, while 36.0% of the patients were diagnosed with ACS with persistent ST-segment elevation. The mean GRACE score was 140±47 (Q1–Q3: 58–259), and management was conservative (without coronary angiography) in up to 16.8% of the patients. In turn, 39.1% presented Killip class>I, while 96.9% showed troponin I elevation in the laboratory tests, and 75.2% had an abnormal electrocardiogram upon admission. With regard to renal function, 11.2% had a history of CKD, and the mean GFR of the overall sample was 75.0±30.8ml/min/1.72m2 (Q1–Q3: 18.8–164.1).
Parathyroid hormone levels and clinical characteristicsOf the total patients included in the study, 41 (25.5%; CI: 18.7–32.3%) presented PTH elevation upon admission (128.8±74.9 versus 43.7±15.6pg/ml; p<0.001). Those with the highest PTH levels (Table 1) were older, and the proportion of women was higher, with more cardiovascular risk factors such as hypertension (80% versus 61%; p=0.025) or dyslipidemia (70% versus 51%; p=0.030), and also a greater frequency of chronic ischemic heart disease (53% versus 35%; p=0.044). The patients with high PTH levels were admitted with a poorer Killip functional class (63% versus 30% in Killip class>I; p<0.001) and had higher GRACE scores (177 versus 126; p<0.001). Likewise, these patients were treated more conservatively during admission, with no adoption of interventional techniques (30% versus 12%; p<0.016). With regard to renal function, the patients with high PTH levels were more frequently affected by CKD (29% versus 5%; p<0.001) and had a poorer estimated GFR (54.3 versus 81.3ml/min/1.72m2; p<0.001). From the laboratory test perspective, the patients with high PTH levels showed lesser concentrations of 25(OH)-D (13.9 versus 19.4ng/ml; p=0.003), more anemia (hemoglobin 12.8 versus 14.3g/dl; p<0.001), greater concentrations of NT-proBNP (2701 versus 669pg/ml; p<0.001), higher levels of acute phase reactants such as CRP (4.7 versus 2.7mg/dl; p=0.004) and fibrinogen (494 versus 449mg/dl; p=0.003), and also significantly higher cystatin C values (1.3 versus 0.9ng/l; p<0.001).
Basal characteristics of the study sample according to parathyroid hormone levels.
| PTH≤79.5pg/ml (n=120, 74.5%) | PTH>79.5pg/ml (n=41, 25.6%) | p | |
|---|---|---|---|
| Demographic data | |||
| Age, years | 63.3 (12.9) | 77.8 (10.8) | <0.001 |
| Male gender | 95 (79.8%) | 25 (61.0%) | 0.016 |
| Cardiovascular risk | |||
| Arterial hypertension | 73 (61.3%) | 33 (80.5%) | 0.025 |
| Diabetes mellitus | 38 (31.9%) | 19 (47.5%) | 0.076 |
| Dyslipidemia | 61 (51.3%) | 29 (70.7%) | 0.030 |
| Active smoker | 49 (41.2%) | 10 (24.4%) | 0.062 |
| BMI, kg/m2 | 28.7 (3.5) | 27.6 (4.5) | 0.282 |
| History of cardiovascular disease | |||
| Chronic ischemic heart disease | 42 (35.0%) | 22 (53.6%) | 0.044 |
| Peripheral arterial disease | 10 (8.4%) | 7 (17.1%) | 0.121 |
| Cerebrovascular disease | 6 (5.0%) | 5 (12.2%) | 0.120 |
| Renal function | |||
| CKD | 6 (5.0%) | 12 (29.3%) | <0.001 |
| GFR MDRD-4 IDMS, ml/min/1.72m2 | 81.3 (29.5) | 54.3 (26.0) | <0.001 |
| Data upon admission | |||
| Killip class >I | 37 (30.8%) | 26 (63.4%) | <0.001 |
| Troponin I elevationa | 116 (96.6%) | 40 (97.5%) | 0.776 |
| “Abnormal” electrocardiogram | 92 (76.6%) | 29 (70.7%) | 0.671 |
| GRACE score | 126.7 (38.3) | 177.9 (49.6) | <0.001 |
| Conservative management (without interventional techniques) | 15 (12.6%) | 12 (30.0%) | 0.016 |
| Laboratory test data | |||
| 1,25(OH)-D, pg/ml | 128.2 (73.8) | 108.9 (86.2) | 0.218 |
| 25(OH)-D, ng/ml | 19.4 (10.5) | 13.9 (7.9) | 0.003 |
| Calcium, mg/dl | 9.8 (7.2) | 8.8 (0.5) | 0.379 |
| Corrected calcium, mg/dl | 9.2 (0.39) | 9.1 (0.4) | 0.074 |
| PTH, pg/ml | 43.7 (15.6) | 128.8 (74.9) | <0.001 |
| Albumin, g/dl | 3.9 (0.4) | 3.6 (0.4) | 0.003 |
| Fibrinogen, mg/dl | 449.4 (139.1) | 494.4 (144.6) | 0.003 |
| Cystatin C, mg/l, median (IQR) | 0.9 (0.26) | 1.3 (0.6) | <0.001 |
| CRP, mg/dl | 2.7 (3.2) | 4.7 (5.0) | 0.004 |
| NT-proBNP, pg/ml, median (IQR) | 669 (2.017) | 2.701 (6.963) | <0.001 |
| Hemoglobin, g/dl | 14.3 (1.9) | 12.8 (1.8) | <0.001 |
25(OH)-D: 25-hydroxyvitamin D; 1,25(OH)-D: 1,25(OH) vitamin D; CKD: chronic kidney disease; GRACE: Global Registry of Acute Coronary Events; BMI: body mass index; NT-proBNP: amino-terminal fraction of brain natriuretic peptide; CRP: C-reactive protein; PTH: parathyroid hormone; IQR: interquartile range; GFR MDRD: glomerular filtration rate.
The patients with calcidiol deficiency (<10pg/ml) presented greater comorbidity, and were older (71.9 versus 65.6 years; p=0.013), with a greater proportion of chronic ischemic heart disease (56.8% versus 35.0%; p=0.022) and chronic obstructive pulmonary disease (18.9% versus 4.1%; p=0.007), and tended to suffer more peripheral arterial disease (18.9% versus 8.1%; p=0.073). In turn, the patients with low calcitriol levels (<48pg/ml) were likewise of older age (74.6 versus 66.3 years; p=0.025).
Parathyroid hormone and other biomarkers in the patients with eventsIn comparison with the individuals that had a favorable outcome, the patients with events had higher concentrations of PTH (86.5 versus 56.2pg/ml; p=0.010) and NT-proBNP (5262.4 versus 1790.6pg/ml; p<0.001). No differences were observed in terms of CRP (4.2 versus 2.8mg/dl; p=0.061), fibrinogen (464.3 versus 461.3mg/dl; p=0.901), calcidiol (17.4 versus 18.1ng/ml; p=0.688) or calcitriol (119.1 versus 125.1pg/ml; p=0.691).
Prognostic implicationsA total of 50 events were documented in the course of follow-up (26 deaths and 24 readmissions due to ACS, of which 14 corresponded to new myocardial infarction and 10 to unstable angina) (period of follow-up of 275 patients-year).
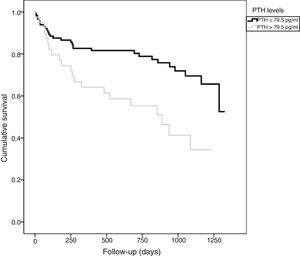
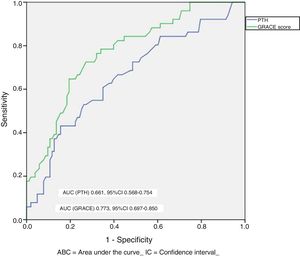
The patients with adverse events had higher circulating PTH values (86.5±78.2 vs. 56.2±35.9pg/ml; p=0.010) and greater NT-proBNP (5262±6545 vs. 1790±3737pg/ml; p<0.001) and CRP levels (4.2±4.9 vs. 2.8±3.1mg/dl; p=0.061). In turn, the patients with high PTH presented an events IR higher than that of the patients with normal PTH levels (IR: 34.9 vs. 13.2 per 100 patients-year; IR ratio: 2.64; 95%CI: 1.5–4.6). In the Kaplan–Meier analysis (Fig. 1) during follow-up, the total adverse events rate was greater in the group of patients with high PTH concentrations (Chi2=10.098; log-rank p<0.001). The GRACE score showed better diagnostic performance (greater area under the curve [AUC]) than PTH in the identification of patients at risk (Chi2=8.0; p for the comparison of areas=0.044) (Fig. 2). The details referred to the diagnostic performance of PTH are shown in Table 2. Of note was the specificity and negative predictive value of the parameter, though with only moderate global efficiency.
Diagnostic performance of parathyroid hormone (PTH) in the identification of patients at risk of the combined event.
| Value and 95% confidence interval | |
|---|---|
| Sensitivity, % | 43 (31–57) |
| Specificity, % | 82 (73–88) |
| Positive likelihood ratio | 2.38 |
| Negative likelihood ratio | 0.69 |
| Positive predictive value, % | 54 (39–68) |
| Negative predictive value, % | 75 (66–82) |
| Global efficiency, % | 69 (62–76) |
The cut-off point recommended by the manufacturer has been used for the estimations. The confidence intervals have been calculated using the Wilson method.
In a Cox model adjusted for the GRACE score, the PTH levels considered both on a continuous basis (HR=1.0; 95%CI: 0.9–1.0; Harrell's C-statistic 0.726) and as a dichotomized parameter (HR=1.1; 95%CI: 0.6–2.2) were not correlated to a poor prognosis. Likewise, neither calcidiol (HR=0.9; 95%CI: 0.6–1.5; Harrell's C-statistic 0.717) nor calcitriol (HR=1.0; 95%CI: 0.4–2.2; Harrell's C-statistic 0.699) was independently associated to a poorer prognosis. In a similar analysis adjusted for the GRACE score referred to the rest of the analyzed biomarkers, none of the latter were seen to be significantly associated to patient outcome. Further tests adjusted for the main comorbidities, in addition to the GRACE score, resulted in no significant changes in the findings (data not shown).
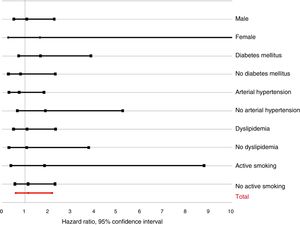
Analysis according to subgroupsFig. 3 shows HR adjusted for dichotomized PTH according to the main subgroups. No interactions were observed with gender (p for the interaction=0.234), diabetes mellitus (p=0.263), hypertension (p=0.605), dyslipidemia (p=0.909) or active smoking (p=0.512). Likewise, no interaction was observed for obesity (p=0.680), prior ischemic heart disease (p=0.987), CKD (p=0.426), prior stroke (p=0.530), peripheral arterial disease (p=0.226), chronic obstructive pulmonary disease (p=0.664), left ventricular systolic dysfunction (p=0.400), or 25(OH)-D concentration (p=0.611).
DiscussionOur study offers the following novel findings: (1) patients admitted to hospital due to ACS often present high PTH levels (approximately one out of every four patients); (2) patients with high PTH levels have more cardiovascular risk factors, suffer more extensive infarctions, with greater risk, with more heart failure, and are more often managed on a conservative basis, with greater associated inflammation, and a poorer outcome after discharge; (3) parathyroid hormone nevertheless was not identified as an independent predictor of poor patient outcome after adjustment for the GRACE scale–thus suggesting that most of the contributed information is already included in a stratification scale commonly used in clinical practice; and (4) calcidiol and calcitriol likewise were not found to be useful instruments for the risk stratification of patients with ACS.
At present, many studies support the relationship between cardiovascular disease and hyperparathyroidism. The presence of high PTH levels has been associated to an increased cardiovascular risk–the latter being largely attributed to an increased prevalence of the classical cardiovascular risk factors, including fundamentally hypertension14 and glucose intolerance.15 The proposed mechanisms underlying hypertension in patients with hyperparathyroidism include greater arterial stiffness in long-standing or severe disease, direct stimulation of the renin-aldosterone system through PTH, and endothelial dysfunction, with an increase in sympathetic activity. Likewise, high PTH levels have been associated to a decrease in insulin sensitivity, with associated hyperinsulinemia.15 In this regard, recent observational studies in our setting, such as that published by Garcia-Martin et al. (2014),6 have retrospectively analyzed patients with asymptomatic primary hyperthyroidism, revealing a high prevalence of obesity (59.9%), diabetes mellitus (25%), hypertension (47.2%) and dyslipidemia (44.4%). The prognostic impact of hyperparathyroidism in relation to cardiovascular disease was evidenced in the study of Vestergaard and Mosekilde (2003),16 who recorded a decrease of approximately 40% in the relative risk of myocardial infarction, stroke and death in patients with surgically corrected primary hyperparathyroidism compared with those individuals treated on a conservative basis.
Despite the above, there is still very weak evidence of the relationship between PTH concentration and the patient prognosis once coronary disease has been established. Recent studies have analyzed the presence of conditioning factors associated to a more adverse clinical profile in patients with stable chronic angina and high PTH levels. For example, it has been seen that the presence of high PTH levels is correlated to more complex coronary disease, with a higher Syntax score and increased calcification (Martín-Reyes et al., 2016),8 or to a suboptimum response to antiplatelet medication secondary to greater platelet reactivity (Verdoia et al., 2016).9 With regard to the prognostic implications in stable patients, high PTH levels have also been related to increased cardiovascular adverse events during follow-up. The study published by Tuñón et al. (2016),10 involving the analysis of patients 6 months after admission due to ACS, found that type 2 diabetics who subsequently suffered more events during follow-up presented higher levels of PTH [71.3 (47.3–106.6) vs. 51.9 (40.8–66.2) pg/ml; p=0.004], fibroblast growth factor-23 [112.0 (59.9–167.6) vs. 68.9 (54.2–93.0) RU/ml; p=0.002] and phosphorus [(3.53±0.71 vs. 3.25±0.50mg/dl; p=0.017)] than diabetics who did not develop adverse events–no significant differences being recorded in non-diabetic patients. In this same line, a small study of 22 patients over 60 years of age admitted due to ACS without ST-segment elevation concluded that the presence of low 25(OH)-D levels and high PTH concentrations identified those individuals with a greater probability of suffering a cardiovascular adverse event (3 patients) during follow-up–the PTH levels being significantly higher in patients with such events.11
In this regard, our study consistently shows that high PTH levels in patients with ACS are associated to a poorer outcome in terms of mortality and readmission due to myocardial infarction or angina, though this association practically disappears on adjusting for the GRACE score–thus suggesting the absence of an independent effect upon the prognosis in this group of patients. The observation of a greater IR of events in patients with elevated PTH levels during follow-up was reasonable, taking into account that these individuals had an increased cardiovascular risk and a larger number of poor prognosis predictive factors than those with normal PTH values upon admission. The patients with high PTH levels presented more cardiovascular risk factors such as hypertension and dyslipidemia, as well as a more frequent history of chronic ischemic heart disease. Our study coincides with previous publications that have analyzed the higher prevalence of such risk factors in this population group,6,7 though with notoriously different prevalences, e.g., in relation to hypertension (80.0% of the population with high PTH levels in our series versus 47.2% in previous registries), diabetes (47.5% of the population with high PTH levels in our series versus 25% in previous registries) or dyslipidemia (71% of the population with high PTH levels in our series versus 44% in previous registries). These findings are probably related to the fact that our population was characterized by greater cardiovascular risk, older age and prior ischemic events, while the patients in the aforementioned studies6,7 were limited to individuals with a diagnosis of asymptomatic primary hyperparathyroidism. From the perspective of renal function, the patients with high PTH levels had a more frequent history of CKD, and presented poorer GFR and higher cystatin C levels. This impairment in renal function has been found to be an important independent prognostic marker in ischemic heart disease–being consistently identified as a risk factor for cardiovascular events and global mortality in high risk populations.17,18 Mention also must be made of the fact that the patients with high PTH levels presented other laboratory test data directly related to an adverse cardiovascular prognosis in the context of ACS, such as a greater presence of anemia, higher NT-proBNP levels, or the presence of greater acute phase reactant concentrations – this being in concordance with the observations of previous studies.19–21
On the other hand, we found that patients with high PTH levels had lower 25(OH)-D concentrations [rho=−0.274; p=0.001]. This observation is interesting, since previous studies have documented the relationship between lowered 25(OH)-D and an adverse outcome in ACS21–24 thereby representing an additional poor prognosis marker in this group of patients. Furthermore, vitamin D deficiency is associated to an increased prevalence of comorbidities in the general population,24 and it has been postulated that vitamin D concentration could reflect the “global health” of an individual.25 This observation is consistent with the results of our study.
LimitationsOur study has some limitations. In effect, its observational design means that there is a risk of confounding bias. On the other hand, the investigation was conceived as being of an exploratory nature, and it therefore cannot be ruled out that studies of greater power might identify prognostic value.
ConclusionsThere was a high prevalence of elevated PTH levels among the patients admitted due to ACS, and the presence of such elevation was associated to a poorer clinical profile and outcome. However, PTH elevation was not identified as an independent predictor of poor prognosis on adjusting for a commonly used scale (GRACE score). In turn, while low calcidiol and calcitriol levels were associated to an increased risk and to more comorbidity, they were not found to be useful in terms of risk stratification.
Authors’ contributionAll the authors have participated in the research work and in the preparation of the article, and have approved the final version of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks are due to the Department of Clinical Analyses, and in particular to Carmen Nieto, for contribution to the determination of parathyroid hormone.
Please cite this article as: Ramos Ruiz P, Jaulent Huertas L, Castañeda Sancirilo M, Martínez Díaz JJ, Clavel Ruipérez G, García de Guadiana Romualdo L, et al. Hormona paratiroidea, calcidiol, calcitriol y riesgo de eventos adversos en pacientes con síndrome coronario agudo. Med Intensiva. 2018;42:73–81.