The COVID-19 pandemic has led to the admission of a high number of patients to the ICU, generally due to severe respiratory failure. Since the appearance of the first cases of SARS-CoV-2 infection, at the end of 2019, in China, a huge number of treatment recommendations for this entity have been published, not always supported by sufficient scientific evidence or with methodological rigor necessary. Thanks to the efforts of different groups of researchers, we currently have the results of clinical trials, and other types of studies, of higher quality. We consider it necessary to create a document that includes recommendations that collect this evidence regarding the diagnosis and treatment of COVID-19, but also aspects that other guidelines have not considered and that we consider essential in the management of critical patients with COVID-19. For this, a drafting committee has been created, made up of members of the SEMICYUC Working Groups more directly related to different specific aspects of the management of these patients.
La pandemia por COVID-19 ha provocado el ingreso de un elevado número de pacientes en UCI, generalmente por insuficiencia respiratoria severa. Desde la aparición de los primeros casos de infección por SARS-CoV-2, a finales de 2019, en China, se ha publicado una cantidad ingente de recomendaciones de tratamiento de esta entidad, no siempre respaldadas por evidencia científica suficiente ni con el rigor metodológico necesario. Gracias al esfuerzo de distintos grupos de investigadores, actualmente disponemos de resultados de ensayos clínicos, y otro tipo de estudios, de mayor calidad. Consideramos necesario realizar un documento que incluya recomendaciones que recojan estas evidencias en cuanto al diagnóstico y el tratamiento de COVID-19, pero también aspectos que otras guías no han contemplado y que consideramos fundamentales en el manejo del paciente crítico con COVID-19. Para ello se ha creado un comité redactor, conformado por miembros de los Grupos de Trabajo de SEMICYUC más directamente relacionados con diferentes aspectos específicos del manejo de estos pacientes.
At the end of 2019, a new virus currently known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused an outbreak of 27 cases at a fish market in Wuhan, China. The virus then spread quickly throughout the world, and on 11 March 2020, the World Health Organization (WHO) declared that the disease produced by SARS-CoV-2 (COVID-19) had become a pandemic.1 By 22 May 2021, over 165 million cases of infection had been confirmed, with close to 3.5 million deaths worldwide.2
Although most cases of COVID-19 are mild or only involve minor symptoms, between 5–10% of all affected individuals require hospital admission and oxygen therapy, and many of them suffer severe respiratory failure needing ventilatory support and admission to the Intensive Care Unit (ICU). In a considerable number of cases the volume of patients requiring admission overwhelmed the capacity of the ICUs, causing COVID-19 to become a serious challenge for healthcare systems throughout the world - including Spain.3–12
At the time when the ICUs were being overwhelmed by the number of patients requiring admission, different studies started to appear in the literature,13–16 not always warranted by sufficient scientific evidence,17,18 recommending different management strategies for a disease in which no effective treatment was available.
Although not even two years have gone by since COVID-19 first appeared, extraordinary efforts by different research groups have produced results from numerous clinical trials and other studies that allow us to establish a series of recommendations based on more solid scientific evidence - though many gaps in our knowledge remain. Some of the mentioned documents have been updated using different methodologies.19,20 We consider it necessary to offer a consensus document including recommendations based on the available evidence, referred to the diagnosis and treatment of COVID-19, but also addressing aspects which other guides have not contemplated and which we feel to be crucial for the management of critical patients with COVID-19.
MethodologyScope and objectivesThe purpose of this document is to offer a number of recommendations based on the available scientific evidence for the diagnosis and management of adults admitted to the ICU due to COVID-19.
It is addressed to medical staff in intensive care, and seeks to be of help both in decision making and in establishing standards of care, while also contributing to organizational planning of the ICU.
Selection of the document drafting committeeThe structure, questions to be answered, and the methodology of the consensus document were defined through coordination of the Infectious Diseases and Sepsis Working Group (Grupo de Trabajo de Enfermedades Infecciosas y Sepsis [GTEIS]) of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias [SEMICYUC]). Subsequently, the coordinators of the working groups centered on the different proposed sections of the document were invited to select the experts who would be in charge of answering the questions raised and to generate possible additional questions as considered opportune.
MethodologyA working team and a coordinator were established for each of the 11 sections.
The scientific evidence available from the start of the pandemic (December 2019) and up until 28 February 2021 was reviewed.
From the coordinating board of the project, and with the agreement of the coordinators of each of the individual sections, in the awareness of the lack of clinical trials in many of the areas considered to be important, priority was placed more on the drafting of a practical document than on rigid methodological considerations.
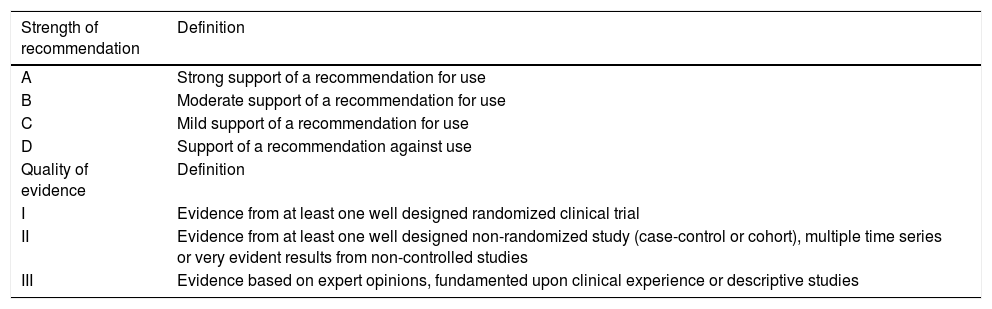
After evaluating and analyzing the available literature, the recommendations were defined by consensus among the members of each working group, followed by review on the part of the rest of the drafting committee. Each recommendation was graded considering the strength of the recommendation and the quality of the evidence (Table 1). The document contains the recommendations issued by each working group. The justification and analysis of the evaluated evidence, as well as some additional recommendations (in general negative or less specific recommendations referred to critical COVID-19 cases) are compiled as electronic supplementary material (ESM).
Grading of recommendations.
| Strength of recommendation | Definition |
|---|---|
| A | Strong support of a recommendation for use |
| B | Moderate support of a recommendation for use |
| C | Mild support of a recommendation for use |
| D | Support of a recommendation against use |
| Quality of evidence | Definition |
| I | Evidence from at least one well designed randomized clinical trial |
| II | Evidence from at least one well designed non-randomized study (case-control or cohort), multiple time series or very evident results from non-controlled studies |
| III | Evidence based on expert opinions, fundamented upon clinical experience or descriptive studies |
The document has been subjected to external review by the members of the SEMICYUC.
RecommendationsDiagnosis (justification of the recommendations can be found in ESM 1)
Question 1. In which critical patients should SARS-CoV-2 be suspected?
We recommend diagnostic testing for SARS-CoV-2 in all patients admitted to the Department of Intensive Care Medicine, particularly in the presence of any respiratory sign or symptom such as cough or dyspnea, or general manifestations such as a rise in temperature, even if of small magnitude. A-III
Question 2. If COVID-19 is suspected, which is the best sample for diagnosing SARS-CoV-2 infection?
We recommend basing the etiological diagnosis of SARS-CoV-2 infection on a sample of nasopharyngeal exudate. In the case of negative results and a strong clinical suspicion, we recommend a second sample, and if a negative result is again obtained, we advise a lower respiratory tract sample – preferably through bronchoalveolar lavage (BAL). A-II
Question 3. If COVID-19 is suspected, which is the best technique for diagnosing SARS-CoV-2 infection (PCR, serological tests, etc.)?
We recommend the use of a nucleic acid amplification test such as reverse transcriptase polymerase chain reaction (RT-PCR) for diagnosing acute SARS-CoV-2 infection. A-II
We suggest repetition of the RT-PCR test in the event of discrepancies between the pre-test probability and the result obtained, preferably using different targets. C-II
We suggest the use of fast antigen testing for rapid decision making, provided there is agreement between the pre-test probability and the result obtained. C-III
We do not recommend the isolated use of serological tests for diagnosing acute SARS-CoV-2 infection. D-II
We do not recommend the use of serological tests for determining the existence of host immunity against SARS-CoV-2. D-II
We suggest serological tests in the event of a strong suspicion of SARS-CoV-2 infection and a repeatedly negative RT-PCR test, particularly in the presence of a delay of >9–14 days from symptoms onset. C-II
Specific treatment (justification of the recommendations can be found in ESM 2)
Question 4. Does antiviral therapy improve the prognosis of critical patients with COVID-19?
We suggest the use of remdesivir in patients with COVID-19 admitted to the ICU who do not require invasive mechanical ventilation, and preferably on an early basis (in the first three days following the microbiological diagnosis). C-II
Recommendations against the use of hydroxychloroquine, chloroquine, lopinavir-ritonavir, interferon β-1a, favipiravir and ivermectin can be found in ESM 2.
Question 5. Is there an inflammatory response specific of SARS-CoV-2 infection?
An analysis of the existence of a specific inflammatory response can be found in ESM 2.
Question 6. Should we administer corticosteroids in critical patients with COVID-19? At what dose? For how long?
We recommend corticosteroid therapy as treatment for seriously ill patients with COVID-19 (need for mechanical ventilation). A-I
We recommend low-dose systemic corticosteroids: dexamethasone 6 mg/d for 10 days (A-I) or hydrocortisone 200 mg/d for 7 days (A-II)
We suggest dexamethasone 20 mg/d for 5 days, followed by dexamethasone 10 mg/d for 5 days in patients with moderate to severe acute respiratory distress syndrome (ARDS) (B-II).
Question 7. Should we try to modulate the inflammatory response with other immunosuppressors/immune modulators in critical patients with COVID-19?
We recommend the administration of tocilizumab (8 mg/kg, maximum 800 mg), associated to corticosteroids, in patients requiring admission to the ICU for respiratory or hemodynamic support, on an early basis (first dose in the first 24 h of admission, with the possibility of a second dose 12−24 h after the first, if the treating physician considers the response to be insufficient). A-II
We suggest the use of sarilumab (400 mg) in patients requiring admission to the ICU for respiratory or hemodynamic support, on an early basis (first dose in the first 24 h of admission), as an alternative to tocilizumab if the latter drug is not available. B-II
We suggest adding baricitinib (4 mg/d, 14 days) in patients receiving remdesivir and high-flow oxygen therapy (HFOT) or noninvasive mechanical ventilation (NIMV), with the aim of shortening the recovery period (the criteria for using remdesivir are those established above: patients not requiring invasive mechanical ventilation, and in the first three days after the microbiological diagnosis). C-I
We do not recommend the use of convalescent plasma to treat patients admitted to the ICU due to severe COVID-19 pneumonia. D-I
Recommendations against the use of ruxolitinib, intravenous immune globulin (IVIG), colchicine, auxora, itolizumab and anakinra can be found in ESM 2.
Coinfections and superinfections (justification of the recommendations can be found in ESM 3)
Question 8. What patients with COVID-19 should start to receive antibiotic treatment upon admission to the ICU?
We recommend early empirical treatment of possible bacterial pulmonary coinfection (strong clinical suspicion, purulent secretions, biomarker elevation, positive antigens, etc.) upon admission to the ICU of patients with COVID-19, since such coinfection is associated to increased mortality. A-III
We recommend daily evaluation of antibiotic treatment adjustment or suspension. A-II
We recommend the early suspension of antimicrobial treatment once coinfection is ruled out. A-III
Question 9. What is the indicated diagnostic strategy in these patients with suspected superinfection during admission to the ICU?
We suggest an early diagnostic strategy and empirical treatment, in view of the high risk of bacterial and fungal superinfection in patients with COVID-19 subjected to mechanical ventilation. A-III
We suggest active microbiological assessment in all patients with prolonged ICU stays (over 7 days) in the event of suspected superinfection. A-III
We suggest lower respiratory tract sampling in patients with COVID-19 suspected to have superinfection in relation to ventilator-associated pneumonia (VAP) or ventilator-associated tracheobronchitis, before starting antibiotic treatment. We suggest the quantitative analysis of distal samples obtained through bronchoalveolar lavage (BAL), mini-BAL or bronchial aspirate - provided these procedures can be carried out safely for both the operator and the patient. If not possible, the alternative would be a lower respiratory tract sample in the form of a quantitative or semi-quantitative tracheal aspirate. A-II
We recommend basing the diagnosis of pulmonary aspergillosis associated to COVID-19 on galactomannan detection in BAL. We recommend BAL with culture and galactomannan determination in patients in which Aspergillus is identified in respiratory samples, with signs of pulmonary superinfection, and also in those with suspected ventilator-associated pneumonia or tracheobronchitis, with negative respiratory sample cultures. A-II
Question 10. How should the healthcare associated infection (HAI) preventive measures be applied in these patients?
We recommend application of the guidelines of the prevention programs (Zero Projects), i.e., Bacteremia Zero, Pneumonia Zero and Resistances Zero, adapted to COVID-19. A-III
Recommendations on hand hygiene and training in prevention programs can be found in ESM 3.
Thrombotic complications (justification of the recommendations can be found in ESM 4)
Question 11. How should thrombotic events be prevented in critical patients with COVID-19?
We recommend that venous thromboembolic disease (VTED) prevention in patients with severe COVID-19 pneumonia be based on low molecular weight heparin (LMWH) instead of unfractionated heparin (UFH). Dose adjustment of LMWH to patient weight, kidney function and platelet count is advised. A-II
We recommend treatment with intermediate LMWH doses in patients with severe COVID-19 pneumonia presenting elevated D-dimer levels (>6 times the upper limit of normal) and/or a sepsis-induced coagulopathy (SIC) score ≥4. B-II
We suggest that full-dose anticoagulation should not be used in patients with severe COVID-19 pneumonia in which no thrombotic event has been diagnosed, except if clinical suspicion is very strong and the patient cannot be mobilized to perform complementary explorations. D-II
We suggest ultrasound screening to discard deep venous thrombosis (DVT) in the lower and upper extremities when D-dimer >2000 ng/mL. We suggest thoracic CT angiography and ultrasound in the presence of strong clinical suspicion of pulmonary thromboembolism (PTE) and/or D-dimer >10,000 ng/mL. B-II
Additional recommendations on the prevention of venous thromboembolic disease in patients with COVID-19 can be found in ESM 4.
Question 12. What is the best diagnostic strategy when PTE is suspected in critical patients with COVID-19?
We recommend CT angiography as the best diagnostic tool when PTE is suspected, both in patients with mild manifestations and in those with severe manifestations of the disease. A-I
Additional recommendations on the diagnosis of PTE in patients with COVID-19 can be found in ESM 4.
Question 13. What treatment is indicated in critical patients with COVID-19 in which PTE is suspected/confirmed?
We recommend anticoagulation of all patients with COVID-19 in which PTE has been confirmed, provided it is not contraindicated. A-I
We recommend the start of anticoagulation when PTE is suspected, when no firm diagnosis can be established, and provided it is not contraindicated. A-III
We recommend systemic fibrinolysis of all patients with COVID-19 in which PTE has been confirmed and who suffer hemodynamic instability, provided it is not contraindicated. A-III
We recommend avoiding fibrinolysis in patients with COVID-19 who suffer ARDS without hemodynamic instability or data indicating PTE. D-III
Additional recommendations on the management of PTE in patients with COVID-19 can be found in ESM 4.
Respiratory management (justification of the recommendations can be found in ESM 5)
Question 14. In which patients can noninvasive respiratory support (NIRS) be considered safe?
We suggest the use of high-flow oxygen therapy as the next step after conventional oxygen therapy, and the guidance of treatment based on the ROX index in adults with SARS-CoV-2 infection and moderate to severe hypoxemic acute respiratory failure (ARF) (p02 < 80 mmHg or Sat02 < 90% with Fi02 > 40%), with no increase in respiratory effort. C-III
We suggest evaluating noninvasive mechanical ventilation in patients with SARS-CoV-2 infection and hypoxemic acute respiratory failure in which high-flow oxygen therapy has failed or is not available, and in the absence of an urgent indication of orotracheal intubation or when the order is to not intubate. This always must be done with close monitoring, in a setting where intubation can be performed safely. C-III
Question 15. In the event invasive mechanical ventilation proves necessary, should a protective ventilation strategy be used?
We recommend a protective ventilation strategy similar to that used in ARDS due to other causes, with a tidal volume of 4−8 ml/kg body weight (predicted), in the case of ARDS secondary to COVID-19 pneumonia. C-II
Once the tidal volume has been adjusted to 4−8 ml/kg body weight (predicted), we recommend monitoring of the plateau pressure, which should not exceed 30 cmH2O. C-II
We recommend maintaining a driving pressure <15 cmH2O. C-II
Question 16. What is the indication of ventilation in prone decubitus in patients with ARDS secondary to COVID-19?
We recommend prone decubitus in all intubated patients with moderate to severe ARDS, provided it is not contraindicated. A-II
We recommend keeping the patient in prone decubitus for at least 16 consecutive hours; this period may be prolonged, conditioned to patient tolerance and response. B-III
There is no evidence to establish recommendations regarding prone decubitus in waking state patients with respiratory failure under conditions of spontaneous ventilation. C-III
Question 17. What is the indication of extracorporeal membrane oxygenation (ECMO) in patients with ARDS secondary to COVID-19?
We recommend veno-venous (VV) ECMO in experienced units or reference centers, in selected patients with severe ARDS and refractory and/or hypercapnic hypoxemic respiratory failure, in the absence of contraindications, when conventional therapies prove ineffective, particularly in the context of prone decubitus. B-II
We recommend cannulation at the patient beside (point of care), in order to minimize the risks for the healthcare staff. B-III
We recommend continued assessment of the risk/benefit ratio of ECMO. B-III
We advocate the usual anticoagulation protocols in patients with COVID-19 subjected to veno-venous ECMO. C-III
Management of sedation and delirium (justification of the recommendations can be found in ESM 6)
Question 18. What is the best sedoanalgesia strategy in critical patients with COVID-19?
We recommend sequential sedation strategies that facilitate adaptation to mechanical ventilation, guaranteeing adequate analgesia and avoiding oversedation. B-III
Question 19. Is there a higher incidence of delirium among critical patients with COVID-19 than in other similar groups of patients?
An analysis of the incidence of delirium associated to COVID-19 can be found in ESM 6.
Question 20. What is the best strategy for controlling delirium in critical patients with COVID-19?
We suggest the best strategy for controlling delirium in critical patients with COVID-19 to be prevention with the ABCDEF-R bundle of measures adapted to patients with COVID-19, and the early detection of delirium using validated scales such as the CAM-ICU and ICDSC. B-III
We suggest treatment based on non-pharmacological measures and dexmedetomidine i.v., especially in patients with hyperactive delirium complicating weaning from mechanical ventilation. B-III
Nutritional management (justification of the recommendations can be found in ESM 7)
Question 21. What is the most advisable nutritional strategy in critical patients with COVID-19?
We recommend identification within the first 24−48 h of admission of possible nutritional risk or the presence of malnutrition based not only on the clinical history but also on some of the available nutritional screening tools, such as the NUTRIC score. A-III
We recommend evaluation of the nutritional and metabolic parameters (proteins, prealbumin, albumin, cholesterol, triglycerides, ion profile, vitamins and oligoelements) in order to avoid possible refeeding syndrome. A-III
In the event of risk of refeeding syndrome, we recommend nutrition to start with half of the calculated calories (hypocaloric diet) and to gradually increase the amount (every 3 days) until the goal is reached (70–80% of the requirements) within 4–7 days. In this period regular controls of the serum levels of phosphorus, magnesium and potassium are required (especially during the first 3 days, when risk is higher). A-III
We recommend adjustment of the energy needs on a daily basis, adapting the regimen according to the type of nutrition used and the stage of the disease. A-III
We recommend the use of simple equations based on body weight and accessible to all the staff caring for these patients. A-III
In non-intubated patients we recommend starting oral feeding as soon as possible. If intake is <60% of energy expenditure for more than two days, it is advisable to add oral hyperproteic nutritional supplements, and if the needs are not met despite this measure, the introduction of complementary enteral or parenteral nutrition is advisable. A-III
In intubated patients, we recommend the start of nutrition in the first 24−48 h of admission, once hemodynamic stability has been achieved, or in the first 12 h after intubation and the start of mechanical ventilation. A-III
In intubated patients, we recommend enteral nutrition as the preferred strategy, even in patients subjected to neuromuscular block or ventilated in prone decubitus. A-III
In patients with a non-functioning digestive tract or unable to tolerate enteral nutrition in which feeding via the digestive tract is unable to cover >60% of energy expenditure for over 48 h, we recommend the start of parenteral nutrition from day four, maintaining trophic enteral feeding whenever possible. A-III
Additional recommendations on the nutritional management of patients with COVID-19 can be found in ESM 7.
Management of cardiovascular complications (justification of the recommendations can be found in ESM 8)
Question 22. What is the best resuscitation strategy in critical patients with COVID-19 and hemodynamic instability?
We recommend exhaustive evaluation of the cause of shock in patients with COVID-19, in order to allow the introduction of adequate treatment and supportive measures. A-II
Additional recommendations on shock resuscitation in patients with COVID-19 can be found in ESM 8.
Question 23. How do we evaluate heart-lung interaction in critical patients with COVID-19 and ARDS?
We recommend the use of dynamic parameters to predict cardiovascular response to volume supply. B-II
We recommend the passive leg elevation maneuver (tidal volume challenge) in patients with ARDS ventilated with tidal volumes ≤8 ml/kg. B-II
We recommend echocardiography as the technique of choice for the initial hemodynamic evaluation of patients with COVID-19 under conditions of shock and for detecting cor pulmonale. The echocardiographic exploration must be performed under conditions of asepsis and sterility in order to minimize the infection risk. A-II
We recommend advanced hemodynamic monitoring in complex situations of shock (such as ARDS and sepsis, ARDS and right ventricular dysfunction) or in cases of difficult stabilization. B-II
Question 24. What are the best therapeutic options in critical patients with COVID-19 and arterial hypertension?
Additional recommendations on the management of arterial hypertension in patients with COVID-19 can be found in ESM 8.
Question 25. What are the clinical manifestations and treatment options in cardiac disorders in critical patients with COVID-19?
We recommend the monitoring of myocardial damage biomarkers (troponin or natriuretic peptide) upon admission and in the course of hospital stay, due to the poorer prognosis of those patients with elevation of such markers. A-II
We recommend the use of echocardiography in the case of elevated myocardial damage biomarkers, as it is safe and useful for establishing the differential diagnosis and allows us to guide and assess the therapeutic strategy on an individualized basis. A-II
Additional recommendations on cardiac disorders in patients with COVID-19 can be found in ESM 8.
Management of neurological complications (justification of the recommendations can be found in ESM 9)
Question 26. What are the priorities for managing the associated neurological manifestations in critical patients with COVID-19?
We recommend a high index of suspicion of potential neurological manifestations (confusion, stroke, encephalopathy, meningoencephalitis and weakness acquired in the ICU) in critical patients with COVID-19. A-III
We suggest the use of noninvasive neurological monitoring. C-III
Additional recommendations on the management of neurological complications in patients with COVID-19 can be found in ESM 9.
Organization (justification of the recommendations can be found in ESM 10)
Question 27. If the usual capacity of the ICU is exceeded, should we modify our regular screening protocol?
We recommend the development of specific screening guides in the context of the pandemic, with a view to defining objective and transparent criteria and thus rationalize the limited available resources and lessen the emotional impact of decision making among the professionals. These guides must have legal backing to protect the professionals and institutions from possible future lawsuits, including public emergency declarations on the part of the government or authorities. B-III
Before introducing these specific screening guides, we suggest that maximum resource expansion be ensured, including patient and/or resource transfers between institutions at local or national level. The guides are to be adapted to the different phases of the pandemic, and their evaluation should include the perspective of society. C-III
We recommend that the objective should be to secure the greatest healthcare benefit possible for the largest number of patients, with the resources available at the time of the decision. All patients with a comparable prognosis should have equal and fair access to the limited resources based on medical and ethical criteria, with no discrimination of any kind. This implies the inclusion of both COVID-19 cases and non-COVID-19 cases in the screening process. B-III
We recommend the individual and dynamic evaluation of all patients, and the use of objective tools to help establish the priorities. Use of the Sequential Organ Failure Assessment (SOFA) score as a criterion for establishing priority in patients with COVID-19 is not advised. A-III
We recommend the definition of specific screening criteria for certain procedures where the resources are greatly limited and the opportunity cost is high, such as extracorporeal support techniques (e.g., ECMO). B-III
We recommend the introduction of psychological support measures to reduce the emotional impact, particularly moral suffering among the professionals, and the creation of multidisciplinary screening teams. B-III
Question 28. If the usual capacity of the ICU is exceeded, how do we increase the number of available beds for critical patients?
We recommend the definition of contingency plans allowing us to expand the structural and professional resources. Resource expansion can be applied at different levels, considering the transfer of resources between units and the use of telemedicine. B-III
We recommend the use of predictive models based on dynamic indicators in different scenarios. A-III
We recommend that expanded ICUs should have the means necessary to ensure quality care, with assignment of the most seriously ill patients to the usual units. A-II
We suggest the development of pyramidal multidisciplinary cooperative models led by specialists in Intensive Care Medicine and nurses with expertise in the care of the critically ill, in those situations where the expansion of professional teams proves necessary. C-III
Question 29. Is the grouping of these patients in open cohort units an option?
We suggest the use of open cohort units in those cases where expansion of the ICU beyond its usual structure is not possible, involving units with the same capacities and functions. C-III
We recommend efforts to avoid the complications associated with organizations of this kind (nosocomial infections, delirium, impossibility of early mobilization protocols, etc.). B-III
We recommend that priority be placed on admission to closed units, with individual rooms, for patients in the awakening stage and undergoing weaning from mechanical ventilation, where the presence of the family of the patient and physiotherapy and rehabilitation pose fewer difficulties - reserving admission to open units for patients subjected to deep sedation. B-III
Personal protection and isolation measures (justification of the recommendations can be found in ESM 11)
Question 30. What personal protection equipment (PPE) is needed in caring for critical patients with COVID-19?
We recommend the use of materials certified according to European Union standards. A-II
We recommend the use of individual PPE made of impermeable, water-repelling material. A-II
We recommend the use of disposable PPE. A-II
We recommend the combined use of a long coat and sealed gloves with good fitting around the neck, wrists and hands. A-II
We recommend the use of FFP2 or FFP3 self-filtering masks. A-II
We recommend the use of FFP3 self-filtering masks when performing aerosol-generating procedures, particularly cardiopulmonary resuscitation. A-III
We recommend the use of integrally fitting or full-face eye protection measures. B-III
We recommend following the clinical practice guides for removing PPE. A-II
We recommend removal of the gloves and coat in a single step. A-II
We suggest double gloving, especially in aerosol-generating procedures. B-II
We suggest cleaning of the gloves with disinfectant before removing the equipment. B-II
We suggest the use of additional verbal instructions for removing PPE. B-II
We suggest the use of tabs to affix and facilitate the removal of masks or gloves. C-II
Question 31. Should systematic vaccination be recommended for the staff working in the ICU?
Vaccination of all the healthcare staff working in the ICU is recommended, provided there are no specific contraindications. A-I
Question 32. What are the required isolation measures for patients of this kind?
We recommend the observation of airborne transmission and contact isolation measures in the care of all patients with COVID-19 admitted to the ICU. A-II
We suggest measures of caution referred to airborne transmission in open units for patients with COVID-19 where procedures involving a high risk of generating aerosols are frequently performed. B-III
Question 33. When can these measures be suspended?
We recommend suspending the isolation of critical patients with COVID-19 from 21 days after onset of the clinical condition, and provided three days have gone by without clinical manifestations. A-III
We recommend two oropharyngeal PCR tests (spaced at least 24 h apart) to confirm that isolation can be suspended in those patients where there are clinical doubts. A-III
Question 34. Can usual family accompaniment be maintained in patients with COVID-19?
We recommend avoiding the isolation of critical patients in the ICU, adopting all the accompaniment and communication strategies available, under safe conditions. A-III
In end-of-life situations, we recommend that the family should be allowed to be present at the patient bedside, explaining the risks of contagion and offering all the PPE means needed to ensure safety. A-III
Financial supportThis study has received no funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Vidal-Cortés P, Díaz Santos E, Aguilar Alonso E, Amezaga Menéndez R, Ballesteros MÁ, Bodí MA, et al., Recomendaciones para el manejo de los pacientes críticos con COVID-19 en las Unidades de Cuidados Intensivos. Med Intensiva. 2022;46:81–89.