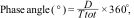Edited by: Rosario Amaya Villar - Unidad de Cuidados Intensivos, Hospital Universitario Virgen del Rocio, Sevilla, España
Last update: December 2023
More infoThis review addresses the phenomenon of "reverse triggering", an asynchrony that occurs in deeply sedated patients or patients in transition from deep to light sedation. Reverse triggering has been reported to occur in 30–90% of all ventilated patients. The underlying pathophysiological mechanisms remain unclear, but “entrainment” is proposed as one of them. Detecting this asynchrony is crucial, and methods such as visual inspection, esophageal pressure, diaphragmatic ultrasound and automated methods have been used. Reverse triggering may have effects on lung and diaphragm function, probably mediated by the level of breathing effort and eccentric activation of the diaphragm. The optimal management of reverse triggering has not been established, but may include the adjustment of ventilatory parameters as well as of sedation level, and in extreme cases, neuromuscular block. It is important to understand the significance of this condition and its detection, but also to conduct dedicated research to improve its clinical management and potential effects in critically ill patients.
Esta revisión aborda el fenómeno "trigger reverso", una asincronía que se presenta en pacientes sedados o en transición de despertar, con una prevalencia en estos grupos del 30% al 90%. Los mecanismos fisiopatológicos aún no están claros, pero se propone el "entrainment" como uno de ellos. Detectar esta asincronía es complejo y se han usado métodos como inspección visual, presión esofágica, ecografía diafragmática y métodos automáticos. El trigger reverso puede tener efectos en la función pulmonar y diafragmática, mediados porbablemente por el nivel de esfuerzo respiratorio y la activación excéntrica del diafragma.El manejo óptimo no está establecido y puede incluir ajuste de parámetros ventilatorios, frecuencia respiratoria, nivel de sedación y en casos extremos, bloqueo neuromuscular. Es importante comprender su significancia, su detección e incrementar la investigación para mejorar su manejo clínico y sus potenciales efectos en los pacientes críticamente enfermos.
Mechanical ventilation (MV) is a life support technique commonly used in the Intensive Care Unit (ICU) in a broad spectrum of patients. Safe and effective ventilation depends on correct interaction between the patient and the ventilator. When ventilation is not adequate, discrepancies arise between the phases of the respiratory cycle and patient breathing effort — a condition known as patient-ventilator asynchrony (PVA).1 Different types of PVA can be classified according to the moment of the ventilatory cycle in which they occur, the underlying pathophysiological mechanism, or the potential consequences.2–4
Recently, reverse triggering (RT) has been described as a type of PVA in which contraction of the respiratory muscles is delayed concerning passive insufflation of the ventilator.5 Reverse triggering can generate different effects at both pulmonary and diaphragmatic level, depending on the magnitude of breathing effort and the clinical condition of the patient.6 The present review describes the epidemiology, detection methods and potential consequences of RT asynchrony, to optimize its management in clinical practice.
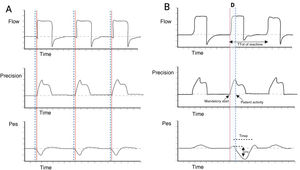
Definition and epidemiology of reverse triggeringReverse triggering corresponds to a specific type of interaction between the patient and the mechanical ventilator, where the diaphragm contracts in a delayed manner or at a certain time after passive insufflation started by the ventilator (Fig. 1),6 without excluding the activation of other respiratory muscles.7
Schematic description of reverse triggering. Panel A) Spontaneous ventilation in VC mode is observed, where the start of patient effort (dotted blue lines) precedes the start of the mandatory ventilation cycle (solid red line). Panel B) RT asynchrony is observed, showing the out-of-phase occurrence of patient effort (Pes negative deflection and dotted blue line) with respect to the start of the mandatory ventilation cycle (solid red line). Abbreviations. VC: volume control; RT: reverse triggering; D: out-of-phase; Pes: esophageal pressure.
This phenomenon was first described in the Intensive Care Unit in 2013, in 8 patients ventilated under volume or pressure assist modes, with the monitoring of respiratory mechanics using an esophageal balloon.5 The authors noted that passive insufflation of the ventilator triggered a patient reflex response resulting in diaphragmatic contraction. It has been seen that RT mainly occurs in patients under deep sedation or who are in transition from controlled ventilation to spontaneous ventilation. Reverse triggering can also manifest in the form of regular patterns that may vary between patients or within the same individual.8
The frequency and form of presentation of this phenomenon varies among the different studies depending on the detection method used, the recording time, the ventilatory mode, the clinical condition of the patient and the unit of analysis used (e.g., number of patients or number of respirations with RT analyzed and recorded in one same patient). The frequency of asynchrony in patients has been variable, though it may represent up to 75% of the respirations; some patients have even presented RT in 90% of their respirations.8,9
PhysiopathologySeveral mechanisms could explain the appearance of RT, as described below.
EntrainmentOne of the main mechanisms proposed to explain RT is "entrainment", which is defined as coupling or alignment between the respiratory center of the patient and the ventilatory cycles programmed in the mechanical ventilator. Entrainment can be observed when there is stable phase coupling between the frequency of stimulation – in this case the passive insufflation ventilatory cycles – and the frequency of the autonomous oscillator (the respiratory rhythm of the patient), acquiring a periodic relationship with different patterns of appearance.
Respiratory entrainment appears to be produced mainly by stretching of the slow adaptation receptors and sustained activation of the Hering-Breuer reflex mediated by the vagus nerve.9 Animal experiments have shown that the abolition of the Hering-Breuer reflex by cooling or sectioning of the vagus nerve impedes this type of interaction.10 It is believed that passive insufflation triggers the Hering-Breuer reflex through stimulation of the stretch receptors in the upper airway, lungs and chest wall, providing afferent feedback to the respiratory center, which subsequently equals the frequency of the external stimulus.11 However, RT has also been described in bilateral lung transplant patients despite sectioning of the vagal afferent pathway — this suggesting that vagal feedback might suffice to activate RT, but is not the only route capable of doing so. The physiology of RT appears to be more complex than believed, and the possibility exists that one or more of these mechanisms may be implicated in a severely ill patient.
Entrainment patternsThe entrainment pattern may be defined as the coupling relationship between the neural respirations and the ventilator cycles. In this regard, 1:1 and 1:2 patterns would be equivalent to a patient respiration in each ventilatory cycle and to a patient respiration every two cycles, respectively. Physiological studies and research in patients subjected to mechanical ventilation have shown the 1:1 relationship to be the most frequent and stable entrainment pattern, which is mainly identified in patients under deep sedation or in transition to awakening with more superficial sedation levels.5 On the other hand, less stable entrainment patterns such as 1:3, 1:4 and 2:3 are evoked with ventilatory patterns different from the intrinsic pattern of the respiratory center. These are more unstable and can be interrupted by irregular patterns. At present, little is known of the factors determining a given pattern or of how entrainment may vary within one same patient.
Phase angleOne of the methods for determining the stability of RT is the analysis of phase angle, which is simply a way to quantify the latency of response of the respiratory center. The phase angle can be calculated as:
where D is the time difference between the start of muscle activity of the patient and the start of mechanical insufflation, and Ttot is the duration of the respiratory cycle. Phase angles between 0° and −180° indicate that patient inspiration starts in the first half of Ttot, and stable 1:1, 1:2, 2:3 patterns can be observed. On the other hand, phase angles between 0° and 180° are obtained when the entrainment ratios are chaotic or less frequent.12 To check the phase angle, we use the coefficient of variation (standard deviation [SD] of the phase angle/mean phase angle × 100). A coefficient of variation of under 15% is considered to be consistent with entrainment, discarding that the observed events are suggestive of respirations produced by chance or other types of asynchrony.13Mechanisms that do not involve the respiratory centerThe evidence of entrainment in lung transplant patients in which the vagal afferent pathways have been sectioned suggests that other mechanisms not mediated by the vagus nerve could play a role in the presence of RT.14
A study in brain death patients has shown that passive insufflations by the ventilator may generate delayed respiratory muscle activation, as occurs in the case of RT. In this context, the absence of respiratory impulses from the brainstem suggests that activation of the respiratory muscles after ventilator insufflation could be mediated by the stimulation of thoracic mechanoreceptors, passive chest movements and/or activation due to elongation of the respiratory muscles.15 Other mechanisms, such as spinal reflexes or the presence of a spinal respiratory pattern generator, potentially could also be implicated.11
Reverse triggering phenotypes and variantsRecent studies have shown that RT may exhibit different phenotypes.16 On analyzing the pressure/volume curve of the Campbell diagram in patients with acute respiratory distress syndrome (ARDS), Baedorf Kassis et al. identified four phenotypes according to the timing of activation and muscle relaxation within the respiratory cycle. The first phenotype is characterized by rapid activation and rapid relaxation (during inspiration or before reaching 50% of the exhalation phase). The second phenotype is characterized by RT with rapid activation in the inspiratory phase and slow relaxation (up to 50% of the exhalation phase) culminating in the expiratory phase. The most frequent phenotype in turn is characterized by rapid activation that produces the greatest contraction peak during the expiratory phase. Lastly, in the fourth phenotype, RT starts and ends during the expiratory phase. The effects of RT may vary in relation to its timing within the respiratory cycle. In this regard, the RT phenotypes that manifest during inspiration could cause increases in inspiratory transpulmonary pressure and volume, while RT occurring during expiration could increase expiratory transpulmonary pressure and incomplete exhalation.
On the other hand, and in contrast to the classical form of RT during controlled mechanical ventilation and deep sedation, some small case series have reported the presence of RT in spontaneous respiratory modes (e.g., pressure support and neurally adjusted ventilatory assist [NAVA]).17 In these cases, the presence of RT could occur due to auto-triggering and airway leakage,18 or due to relaxation of the expiratory muscles that induce contraction of the inspiratory muscles, simulating "pseudo-reverse triggering".19,20 These RT variants require confirmation through future research.
Reverse triggering detection methodsThe detection of RT is crucial for its optimization. This is complex, however, and requires training. Table 1 summarizes the different detection modalities.
Principal reverse triggering detection methods.
| Method | Identification | Advantages | Disadvantages |
|---|---|---|---|
| Visual inspection | ✓ Absence of deflection in the pressure-time curve of the airway at the start of pressurization. | ✓ Accessible in clinical practice using the curves of the mechanical ventilator. | ✓ Need for training. |
| ✓ Reduced peak expiratory flow compared with other respirations. | ✓ No additional equipment required. | ✓ Difficult to detect without double cycling. | |
| ✓ Deformation of the expiratory flow curve (lack of normal exponential decrease). | |||
| ✓ Volume control mode: variation in pause pressure compared with other respirations in the absence of airflow leakage. | |||
| ✓ Pressure control mode: pressure and flow drop during the inspiratory phase. | |||
| Esophageal pressure (Peso) | ✓ Positive change in the Peso signal at the start of respiration, followed by a negative swing in the Peso signal after the start of respiration. | ✓ Detection of the start of contraction of the inspiratory muscles. | ✓ Additional equipment required. |
| ✓ Allows the distinction of reverse triggering phenotypes (Campbell diagram). | ✓ Invasive method (requires insertion of an esophageal catheter). | ||
| ✓ Allows determination of phase angle. | ✓ Expertise in interpretation is required. | ||
| Electrical activity of the diaphragm (EAdi) | ✓ Absence of EAdi at the start of pressurization, followed by an increase in EAdi signal after the start of pressurization (peak > 1 µV). | ✓ Detection of the start of contraction of the inspiratory muscles. | ✓ Additional equipment required (catheter and specific ventilator). |
| ✓ Allows determination of phase angle. | ✓ The electrical activity of the heart may generate artifacts in the EAdi signal (false positive readings). | ||
| Ultrasound | ✓ Passive movement of the diaphragm (elevation) due to lung insufflation. | ✓ Precise detection of the start of contraction of the diaphragm. | ✓ Operator-dependent technique. |
| ✓ New elevation of the diaphragm (in M mode), corresponding to involuntary contraction. | ✓ Technology available in clinical practice; noninvasive. | ✓ Difficulty synchronizing the curves (mechanical ventilator and ultrasound). | |
| ✓ A relatively new method, requiring validation. | |||
| Automated detection | ✓ According to the method used (ventilator curves, Peso, EAdi). | ✓ Continuous monitoring. | ✓ Technology not available in clinical practice. |
| ✓ Recordings of longer duration. | ✓ Off-line data analysis. | ||
| ✓ No healthcare professional is required during detection. | |||
| ✓ Potential usefulness in clinical decision-making (big data). |
The visual inspection of ventilator curves is the most commonly used way to assess the interaction between the patient and the ventilator in clinical practice. However, the capacity of the different healthcare professionals to detect dyssynchrony from the ventilator curves is deficient and scantly feasible due to time restrictions, and the main problem is the poor reproducibility between evaluators.21,22 Nevertheless, several features allow us to identify the presence of RT and distinguish it from other spontaneous breathing efforts or types of dyssynchrony.
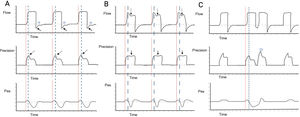
Independently of the ventilatory mode used, we need to establish how it starts the ventilatory cycle (whether started by the patient or by the ventilator). In order for RT to be considered as such, the cycle must be mandatory in the absence of signs of patient effort. The next step is to identify variations in pressure or flow, depending on the ventilatory mode. In volume control - continuous mandatory ventilation (VC-CMV) mode, we can observe variations in the pressure curve, such as a decrease in peak pressure, as well as loss of the expiratory flow peak with a rise in flow in the expiratory phase in the flow-time curve. If there is a programmed pause pressure, it may be altered by eliminating it or modifying its form (Fig. 2A). Likewise, in pressure control — continuous mandatory ventilation (PC-CMV) mode, we can identify variations in the flow-time curve; for example, a rise in flow during the inspiratory phase time interval in the decelerated flow, indicating a contraction of the diaphragm, which at the same time may or may not correspond to a mild drop in the pressure-time curve. In the flow-time curve we can observe an amputation of the peak expiratory flow and a rise in flow in the first third of the cycling phase (Fig. 2B). It is important to note that if contraction of the diaphragm is powerful enough and close to the end of the inspiratory cycle, it may give rise to a new trigger independently of the ventilation mode - a phenomenon known as double cycling caused by RT (Fig. 2C).
Detection of reverse triggering by visual inspection. Panel A) Spontaneous ventilation in VC mode is observed, with the appearance of RT of middle activation and late relaxation, with 1:1 pattern; the red lines indicate the start of mandatory ventilation, and the broken blue lines indicate the response of the diaphragm. In the flow-time curve, the solid arrows indicate amputation of the expiratory flow; the star indicates attempted flow positivity during the expiratory phase. In the pressure-time curve, the broken arrows indicate the drop in peak pressure. Panel B) Ventilation in PC mode is observed, with the appearance of RT of middle activation and late relaxation, with 1:1 pattern; the red lines indicate the start of mandatory ventilation, and the broken blue lines indicate the response of the diaphragm. In the flow-time curve, the broken arrows show an increase in flow during deceleration; in the pressure-time curve, the solid arrows show a slight drop in airway pressure. Panel C) Ventilation in VC mode is observed, with the appearance of RT of middle activation and late relaxation, inducing double cycling; the red lines indicate the start of mandatory ventilation, and the broken blue lines indicate the response of the diaphragm. In the pressure-time curve, the star indicates the double cycling. Abbreviations. VC: volume control; RT: reverse triggering; PC: pressure control; Pes: esophageal pressure.
The reference standard for the identification of RT remains the use of the esophageal balloon (Peso), or some other method that identifies the timing of the activation of the inspiratory muscles, such as the electrical activity of the diaphragm (EAdi). These recordings are very useful, since they can detect dyssynchrony by comparing the time of occurrence of the changes in Peso or EAdi with the airway pressure and flow-time curves in each ventilatory cycle. Using Peso, we detect RT through the negative shift of the esophageal pressure following the start of the mandatory ventilatory cycle. Likewise, delayed activation in the EAdi curve could be interpreted as RT.23 Both types of catheters have been used to detect RT, though their utilization in clinical practice remains limited, since advanced training and technical expertise in acquiring the signals are required.5
Diaphragmatic ultrasoundDiaphragmatic ultrasound is an emergent noninvasive technique for the detection of asynchronies. Direct observation of the diaphragm through its muscle excursion allows us to identify the moment of contraction, which together with visualization of the mechanical ventilator curves can serve to identify RT.24,25 Although this is a promising technique, its use requires experience, and synchronization between the ventilator data and the ultrasound tracing is one of the main challenges.
Automated detection methodsThe main interest in automatization of the detection of asynchronies is driven by their high incidence and the need for very prolonged observation periods. The methods described above usually provide only point or retrospective observations. In the case of RT, five automated methods have been evaluated to date and which use information from the ventilator curves,26 Peso27 and EAdi.9,28 The algorithms used are based on standards developed from the knowledge of asynchronies, and more recently on the use of computational neural networks.29 A summary of the different algorithms and models evaluated, as well as of their diagnostic accuracy in detecting RT, is provided in Table S1 (see electronic Supplementary material).
Clinical implications of reverse triggeringPotential effects of RT have been postulated at both pulmonary and diaphragmatic level, though its association with patient outcomes remains unclear.
Reverse triggering and pulmonary consequencesDifferent mechanisms could explain how RT may be harmful to the lungs. Firstly, superimposing spontaneous effort on ventilator respiration exerts an additive effect upon the transpulmonary pressure in charge of distending the lung parenchyma, and therefore on the resulting tidal volume (VT), mainly in pressure control modes where flow is variable. Baedorf Kassis et al. showed that RT can increase VT on average from 51 to 128 ml, and transpulmonary pressure between 3–7 cmH2O, depending on the phenotype when compared with passive respirations.16
Secondly, the occurrence of RT could be associated with double cycling and excessive tidal volume. During RT, the inspiratory effort of the patient may persist beyond the end of the inspiratory phase programmed by the ventilator. If this effort is sufficiently deep and prolonged, it may lead to double cycling, which is the occurrence of two consecutive inspirations without an expiration in between — resulting in increased VT.30 Observational studies have found that 35% of all double cycling events may be caused by RT, and that this could result in an increase of up to approximately 200 ml in extra VT.30–32
Thirdly, during respirations with RT, we may encounter a phenomenon known as pendelluft, characterized by an exchange of air from the non-dependent pulmonary zones to the dependent zones, causing abrupt tissue deformation and potentially excessive local stretching of the implicated dependent zone.33 Yoshida et al., using electrical impedance tomography in an animal model of lung injury, demonstrated that the pendelluft phenomenon can occur during RT. These investigators observed that air exchange from the non-dependent lung towards the more dependent zones can cause local overdistension up to two times greater than the distension observed in the same region during passive ventilation.34
Lastly, and in contrast to the potentially harmful impact, the presence of RT could have a beneficial effect on the maintenance of end-expiratory volume due to eccentric activation of the diaphragm. In an experimental model of acute respiratory failure, Pellegrini et al. studied the impact of diaphragmatic activation during expiration and found that expiratory diaphragmatic contraction reduces collapse and increases aeration of the lung in comparison with controlled mechanical ventilation without respiratory muscle activity.35 These results suggest that the diaphragm could exert a control effect upon expiration, helping to preserve lung volume at the end of expiration.
Reverse triggering and diaphragmatic consequencesDuring RT, the inspiratory effort of the patient is activated by the ventilator, and in most instances, part of the patient effort takes place in the expiratory phase. During the expiratory phase, the inspiratory muscles (e.g., the diaphragm) elongate as the lung volume decreases. Thus, activation of the inspiratory muscles results in muscle activation during their elongation (eccentric contraction).36,37
The impact of these eccentric contractions of the diaphragm upon its function and structure could run in opposite directions. On one hand, RT could contribute to preventing disuse and atrophy of the diaphragm, since eccentric contraction is characterized by the generation of greater forces than other types of contractions in the face of a certain angular velocity. Furthermore, it has been shown that eccentric contractions require less motor unit activation and consume less oxygen for generating muscle force than concentric contractions.38,39 On the other hand, RT may prove harmful for the diaphragm when the contractions take place repeatedly during the expiratory phase concomitant to the decrease in lung volume and stretching of the inspiratory muscles. Studies in animal models have shown that intense muscle contraction during elongation is associated with a decrease in diaphragmatic contractility and the capacity of the diaphragm to generate pressure.40 Likewise, in an experimental model of acute lung injury, the occurrence of RT with high breathing effort was associated with significantly less diaphragmatic function and a high proportion of abnormal muscle fibers (e.g., muscle damage) in comparison with passive mechanical ventilation without breathing effort.41
Reverse triggering and outcomes in critically ill patientsThe impact of RT on patient-centered outcomes is a current focus of study and research. Several observational studies have evidenced that RT may be associated with some positive outcomes.
Mellado-Artigas et al. studied the incidence of RT in 39 subjects during the first 24 h of mechanical ventilation, using the measurement of EAdi. Interestingly, on dividing the cohort of patients according to the median occurrence rate of RT, they found that those patients with a higher incidence of RT had better oxygenation and more probabilities of progressing towards assist mode ventilation or of being extubated in the next 24 h than patients with a low frequency of RT.9 On the other hand, Rodríguez et al., in an epidemiological study on patients with ARDS, found RT to be associated with a decrease in in-hospital mortality (hazard ratio 0.65; 95%CI: 0.57−0.73), thus suggesting that it may be a marker of improved outcomes in patients subjected to mechanical ventilation.42 At present, larger studies are being carried out to better understand the potential impact of RT and the clinical outcomes in critically ill patients (NCT 03447288).
Approach to the management of reverse triggeringThere currently are no solid data to establish which strategy is best for dealing with RT. In clinical practice, the approach requires an understanding of the factors that condition the appearance of the phenomenon and the consequences it may have during interactions of this kind, as commented in the preceding section. As an example in this regard, a high frequency of RT associated with excessive breathing effort or causing double cycling should be suppressed. Adequate evaluation of the level of breathing effort through noninvasive measurement methods at the patient's bedside, such as occlusion pressure in the first 100 ms (P0.1) and the change in airway pressure during an expiratory pause (ΔPocc), are strongly recommended for discriminating a potentially harmful effect of RT.43,44
Some ventilatory variables such as tidal volume and respiratory frequency could have an impact on the occurrence of entrainment. Hence, modification of the ventilatory parameters could be an interesting strategy for inducing or abolishing RT. Likewise, factors associated with the patient background disease and level of sedation could also influence the incidence of RT.
Programming the respiratory frequency in the mechanical ventilator (oscillator frequency) and its relation to the intrinsic frequency of the patient directly affect the occurrence of entrainment. Studies in anesthetized patients have shown that the presence of entrainment is seen when the ratio between the frequency established by the ventilator and that of the patient is close to one.10 Similarly, experimental evidence on RT has shown that a ventilator frequency adjusted to between 2–5 respirations above the intrinsic frequency of the animal facilitates the occurrence of entrainment.45 Hence, in patients with an active respiratory center and intrinsic respiratory frequency, a decrease in ventilator frequency contributes to allowing the patient to assume part of the breathing effort. Rodríguez et al. showed that a 5-point decrease in respiratory frequency is associated with a mean reduction of two RT events per minute. A larger number of double cycling events was observed, however. On the other hand, the increase in respiratory frequency did not result in changes in the incidence of RT.42 The impact of adjustment to the programmed frequency in the absence of an intrinsic respiratory frequency is not known, and is more complicated to define. It is likely that other ventilatory variables and patient-related factors such as vagal afferent pathways, activation of the respiratory center and level of consciousness may play a role.45
The programming of tidal volume may also play an important role in the occurrence of RT, though the evidence remains controversial. Different clinical studies have described the presence of RT in the context of protective mechanical ventilation with tidal volumes of close to g mg/kg ideal weight.6 In addition, in a porcine model of acute lung injury, a decrease from 10 ml/kg to 7 ml/kg proved successful in inducing RT in all the animals.41 In adult patients with ARDS, the occurrence of RT was independently associated with lower tidal volumes.42 Based on the current evidence, an increase in tidal volume and subsequently in minute volume could abolish RT due to the effect upon the Hering-Brauer reflex, the lowering of arterial CO2 and a decrease in the magnitude and duration of inspiratory effort.46
It is important to note that the impacts of tidal volume and respiratory frequency cannot be viewed as isolated effects but rather as an interaction affecting minute ventilation, and which must be taken into account when addressing RT.
In addition, in patients with an intrinsic respiratory frequency who are in the transition phase from deep sedation to more superficial sedation, a decrease in sedation level could facilitate participation by the patient, activating the respiratory center and accelerating the transition to spontaneous ventilation, with a decrease in the incidence of RT. At present, the impact of the different sedative drugs upon the occurrence of RT is unclear. Lastly, neuromuscular blockers may be used in the presence of RT with excessive effort and double cycling, specifically when it cannot be abolished by modifying the ventilatory parameters or adjusting sedation.47Table 2 describes the main unresolved issues referred to RT, and which represent opportunities for future research in this field.
Unresolved issues in reverse triggering.
| How and which ventilatory parameters influence the incidence and magnitude of reverse triggering? |
| What are the pathophysiological mechanisms underlying the occurrence and different patterns of reverse triggering? |
| How do sedation level and the different sedative drugs interact with the incidence and magnitude of reverse triggering? |
| What is the effect of reverse triggering on lung and diaphragm function and structure in critically ill patients? |
| What is the impact of the incidence and magnitude of reverse triggering upon the patient outcomes? |
Reverse triggering asynchrony is a complex, frequent and often underdiagnosed phenomenon in patients subjected to mechanical ventilation. The underlying pathophysiology and causal mechanisms are currently not known, though a reflex mechanism mediated by vagal activity is the main proposed hypothesis. Reverse triggering can generate opposite effects at pulmonary and diaphragmatic level, depending on the level of breathing effort and the eccentric contractions of the diaphragm. Different variables such as respiratory frequency, VT and sedation are the purported main modulators of the incidence of RT. Nevertheless, further research is needed to improve our understanding of RT and its relation to patient outcomes.
Financial supportNone.
Conflicts of interestThe authors declare that they have no conflicts of interest.
L. Felipe Damiani, partial support from the Agencia Nacional de Investigación y Desarrollo (ANID), Project Fondecyt Regular 2022 / Folio 1220853.