Refractory hypoxemia is an extremely complex condition with a high morbidity-mortality rate. This clinical situation represents an advanced process encompassed within the so-called acute respiratory distress syndrome (ARDS), defined by the American-European Conference of 19941 with the purpose of reaching consensus on a series of homogeneous criteria. Acute respiratory failure (ARF) is defined on the basis of clinical, radiological and blood gas parameters. Acute lung injury (ALI) in turn is defined as a PaO2/FiO2 ratio of under 300mmHg, and in ARDS it is taken to represent a ratio of under 200mmHg. Recently, in the year 2011, experts gathered in Berlin and redefined the classification (the “Berlin definition”),2 improving stratification and mortality prediction of the syndrome, but without clarifying other factors, such as the role of positive end-expiratory pressure (PEEP), or the physiopathology or etiology of the process. The term ALI has disappeared from this classification and the condition is now classified according to the PaO2/FiO2 ratio for an established PEEP as mild, moderate or severe ARDS (PaO2/FiO2 200–300 with PEEP≥5; PaO2/FiO2≤200 with PEEP≥5, and PaO2/FiO2≤100 with PEEP≥10, respectively).
Mechanical ventilation (MV) intrinsically implies lung aggression as described in a number of studies, such as those published by Amato et al.3 and Brochard et al.,4 where the use of lung protecting ventilatory maneuvers has been advised. Based on these data, the study made by the ARDS Network5 in the year 2000 was able to demonstrate a decrease in mortality among patients with ALI/ARDS subjected to lung protecting ventilation. This strategy aimed to reduce ventilator-induced lung injury (VILI), and was based on the avoidance of alveolar overdistension (volutrauma) and cyclic opening and closing of the alveolar units (atelectrauma). Later studies corroborated this strategy, even in patients without lung injury criteria. This was the case of the work published by Determann et al.,6 who demonstrated an increase in the cases of ALI among patients ventilated with a tidal volume (TV) of 10ml/kg ideal body weight (ml/kg IBW) versus 6ml/kg IBW, to the point of having to interrupt the study prematurely. The study published by Needham et al.7 in 2012 analyzed survival after two years among patients with ALI subjected to ventilation with lung protecting measures. These authors recorded a mortality rate of 64% in the first two years. Compliance with the lung protecting measures in 50% of the cases implied a decrease in absolute mortality risk from two years of 4%, versus 8% when compliance was 100%. In contrast, the relative mortality risk increased 18% for every 1ml/kg IBW rise in TV.
Under these premises, any case of acute respiratory failure which under lung protecting measures persistently maintains PaO2/FiO2<100 or plateau pressure (Pplat)>30cmH2O can be classified as refractory hypoxemia.8 Once the diagnosis has been established, evaluation is required of different therapeutic measures that act upon different aspects of lung physiopathology. The objective of this review is to describe the therapies designed to treat hypoxia and improve survival.
Ventilatory optionsMechanical ventilation is the cornerstone of treatment and comprises different ventilatory modes and/or maneuvers that aim to improve the effective gas exchange surface: PEEP level, recruitment maneuvers, pressure regulated ventilation modalities, inverse inspiration-expiration ratio, airway pressure release ventilation (APRV), and high-frequency oscillation ventilation (HFOV).
Positive end-expiratory pressure and recruitment maneuversLung disease is characterized by heterogeneous distribution between healthy alveolar units and units with different degrees of alveolar collapse, which globally reduce the surface available for gas exchange. The application of pressure to the respiratory system can decollapse the damaged alveoli. The main difficulty, however, is to reach a sufficient level of pressure to recruit (decollapse) the diseased alveolar units while simultaneously avoiding cyclic opening and closing (atelectrauma), overdistension of the healthy alveoli, and adverse hemodynamic effects (alteration of the ventilation–perfusion ratio and of cardiac output).
There are two main maneuvers for securing the greatest possible surface for gas exchange, distinguished by their intensity and duration of application. Positive end-expiratory pressure (PEEP) applies continuous pressure during ventilation, with slow and progressive recruitment, while recruitment maneuvers (RMs) involve high pressures maintained for short periods of time, with recruitment of as many collapsed alveoli as possible.
In recent years, the debate regarding PEEP has focused on the application of moderate PEEP versus high PEEP. The results show an improvement of PaO2/FiO2, with no influence upon survival. The ALVEOLI study9 compared low volume-controlled ventilation in two PEEP regimens, with the observation of improved oxygenation and respiratory mechanics in the high PEEP group. However, there were no differences in mortality, days without mechanical ventilation or in the development of barotrauma–though this possibly could be explained by the existence of a certain imbalance between the groups. In this same line, Meade et al.10 compared volume-controlled ventilation (moderate PEEP) versus pressure-controlled ventilation (PEEP high), and observed improvements in refractory hypoxemia and death due to hypoxemia in the high PEEP group, though without differences in either global mortality or the incidence of barotrauma. The study published by Mercat et al.11 in turn compared low volume-controlled ventilation in a standard group and in a group with PEEP elevation to a plateau pressure of 28–30cmH2O. Not only were no benefits observed, but adverse effects were even recorded in the high PEEP group with mild respiratory failure.
Gordo-Vidal et al. reviewed the effect of different PEEP levels in four studies of high methodological quality among the 12 studies regarded as relevant.12 The authors selected the studies of Amato et al.,13 Ranieri et al.,14 Brower et al.9 and Villar et al.,15 and concluded that PEEP level did not affect either mortality or the incidence of barotrauma. The same analysis, without considering the ALVEOLI study published by Brower et al.,9 yielded significant reductions in mortality and barotrauma (RR=0.6; 95%CI 0.4–0.8, and RR=0.2; 95%CI 0.1–0.7, respectively). The difference was based on the fact that in these three studies PEEP was adjusted according to identification of the lower inflexion point of the ascending arm of the pressure/volume loop.
In sum, on the basis of the described studies, it can be concluded that the use of high PEEP to increase oxygenation affords better results. However, PEEP elevations that do not result in an increase in alveolar surface imply increases in transpulmonary pressure, which in turn is related to VILI.16 PEEP monitoring according to P-V loops or the “stress index” is advised in order to avoid deleterious hemodynamic or pulmonary effects.
Recruitment maneuvers expand the effective gas exchange surface through an intense and transient increase in transpulmonary pressure, resulting in the recruitment of collapsed alveoli. Studies such as that published by Oczenski et al.17 report temporary improvements in blood gas parameters. Pressures of 50cmH2O were generated during 30s, with the improvement of oxygenation for 30min. The same results were obtained by Meade et al.,18 with adverse effects, such as hypotension or barotrauma. On the other hand, the PEEP required after RM can be adjusted by identifying the inflexion point of the ascending arm of the pressure–volume curve, as in the study of Amato et al.,13 or by desaturation in the context of progressive PEEP reduction, as in the study published by Girgis et al.19 The review carried out by Fan et al.20, involving 40 studies in patients with ALI, confirmed the temporary improvement of patient oxygenation, with fewer adverse effects than in other articles. The authors concluded that the procedure should not be regarded as a routine measure but as an individualized option in patients in the context of life-threatening situations.
Recruitment maneuvering is the subject of debate, offering transient effects, and with no established optimum methodology, timing or frequency of application. We hope that the OLA multicenter study will offer clarifying results in the near future.
Ventilatory techniquesPressure-controlled ventilationPressure-controlled ventilation (PCV) is a ventilatory option in cases of refractory hypoxemia, since it can improve hypoxemia without adding further risks–though it does not modify patient survival. Esteban et al.21 randomized 79 patients two treatment arms, volume-controlled ventilation and pressure-controlled ventilation, and found no differences in blood gas parameters, ventilatory mechanics or in the number of organ failures.
Since this ventilation mode is applicable not only in refractory hypoxemia, the reader is advised to consult the corresponding topic in the series.
Inverse inspiration-expiration ratioMechanisms have been evaluated that increase mean lung pressure. Prolongation of the inspiratory phase until exceeding the expiration time, inverting the ratio, may be one such mechanism. Although feasible under any ventilation mode, it traditionally has been used in pressure-controlled ventilation, resulting in a decrease in peak pressures and improving ventilation and oxygenation, etc.22
However, over time, no clear benefits of this technique have been reported with respect to conventional ventilation modes. An increased frequency of asynchronization is observed, requiring increased sedation or even relaxation. The method increases the risk of air trapping, with the possibility of hemodynamic deterioration. Mercat et al.23 compared volume-controlled ventilation (VCV), traditional pressure-controlled ventilation, and pressure-controlled inverse-ratio ventilation (PC-IRV). The ventilation (PaCO2: VCV 45±5mmHg, PCV 43±5mmHg, PC-IRV 39±4mmHg; p<0.05) and pressure parameters in the respiratory system improved in the inverse-ratio group, though without changes in oxygenation. However, a worsening of the cardiac index (3.3±0.2 versus 3.7±0.2l/min/m2; p<0.05) and of O2 transport was noted (DO2 424±28 versus 469±38ml/min/m2; p<0.05).
The lack of clear benefits has lessened its clinical use, at least in conventional pressure-controlled ventilation modes.24
Airway pressure release ventilationThe combination of pressure-controlled ventilation and the inverse inspiration-expiration ratio (inverse I:E) resulted in airway pressure release ventilation (APRV).
APRV is a pressure-controlled ventilation mode in which a “low pressure” and a “high pressure” are established. In essence, it is equivalent to PCV, though with two fundamental differences. On one hand, the ventilator is equipped with an active expiratory valve that allows spontaneous patient breathing in any of the pressure phases, and on the other, the duration of the “high pressure” phase is always longer than that of the “low pressure” phase–this being equivalent to an inverse I:E ratio. Spontaneous ventilation lessens the need for sedation and vasoactive drugs,25 increases recruitment of the dependent zones, and improves the V/Q ratio and oxygenation.26,27 In turn, the pressure release phases (low pressure phase) resolve the hemodynamic problems.26
In a series of 24 patients, Putensen et al.26 compared APRV with spontaneous breathing, APRV without assist capacity and pressure support ventilation (PSV), divided into two arms: equal total inspiratory pressure12 or equal minute volume.12 The comparison of APRV with and without spontaneous breathing showed significant improvements in the spontaneous breathing arm referred to cardiac index, oxygenation and the V/Q ratio, with a decrease in pulmonary vascular resistance. Although PSV represents a partial ventilation mode, it did not improve the cardiac index or the V/Q ratio versus APRV without assistance. The authors concluded that spontaneous breathing in APRV offered an advantage in terms of ventilation, oxygenation and cardiovascular hemodynamics, basically as a result of the promotion of activity and recruitment of the dorsal pulmonary regions. In 2001, the same group25 compared APRV versus PCV during the first 72h of mechanical ventilation, in a series of 30 patients. Strict control of all the variables was ensured, and the difference between the two groups was the presence of spontaneous breathing in APRV versus its total absence (under relaxation) in the PCV group. Analyses were made of the blood gas, respiratory and hemodynamic parameters in the first 10 days after admission. The APRV group showed significant increases in compliance, PaO2, V/Q ratio and cardiac index versus the PCV group, as well as a lesser need for sedation and vasoactive drugs. In this same line, Varpula et al.28 conducted a similar study in 58 patients, comparing APRV versus synchronized intermittent mandatory ventilation with pressure support (SIMV-PS). They analyzed the evolution of the blood gas, respiratory and hemodynamic parameters, as well as the days without MV and the mortality rate after 28 days. The study was considered futile and was stopped before reaching the estimated 80 patients, since the results of the interim analysis showed no differences between the groups. The blood gas and hemodynamic results showed no significant differences, in the same way as the days without MV (APRV: 13.4±1.7 days versus SIMV: 12.2±1.5 days) and mortality after 28 days (APRV 17% versus SIMV 18%). The study presented a series of elements that complicate interpretation of the results. On one hand, there was restriction of the maximum spontaneous respiratory frequency for both modes–spontaneous ventilation release being one of the key and differentiating characteristics in the physiopathology of APRV. On the other hand, two confounding factors were introduced that could have influenced the results to some degree, namely utilization of the prone position and of methylprednisolone.
The study published by Neumann et al.29 analyzed the possible adverse effects of APRV. The patients with long expiratory times, as in those with obstructive lung disease, showed a progressive increase in auto-PEEP on reducing the duration of the low pressure phase. It was even noted that spontaneous breathing could result in TV and changes in pleural pressure (sometimes of great magnitude)–this being associated to high transpulmonary pressures and an increased risk of VILI.
To date, none of the studies have shown increased patient survival. In contrast, improvements in hemodynamics and ventilation mechanics can be assumed, though with risks associated to application of the technique. In conclusion, while representing a ventilation alternative, routine application of the procedure cannot be recommended. Furthermore, when used, strict control of the tidal volumes and generation of auto-PEEP is required.
High-frequency oscillation ventilationHigh-frequency oscillatory ventilation (HFOV) is a ventilatory technique characterized by the application of a respiratory frequency of >100rpm, and which is expressed in Hertz (Hz)(generally 3–15Hz). The combination of FiO2 and the mean pressure generated in the respiratory system determines oxygenation. This mean pressure is the result of a minimum pressure oscillation (ΔP) that generates TV values below the dead space, but sufficient to maintain adequate ventilation. Much of the generated pressure is attenuated in the main airway; as a result, the volume and pressure reaching the alveoli are so low that alveolar recruitment is allowed without causing overdistension or cyclic alveolar collapse-opening phenomena. The result is improved gas exchange, maintaining lung protecting measures.
The literature describes this modality as effective and safe in relation to oxygenation and ventilation. Derdak et al.30 conducted a multicenter study comparing conventional ventilation (pressure-controlled ventilation) with HFOV in 150 patients with criteria of ARDS. The primary endpoint–the reduction of mortality after 30 days–failed to reach statistical significance (HFOV 37% versus PCV 52%, p=0.1). The results generated controversy, since the lung protection guidelines of the ARDS Network were not followed,1 and this could have explained the high mortality in the control group. However, significant improvement of the PaO2/FiO2 ratio was observed in the HFOV group in the first 16hours (205 versus 143mmHg; p<0.007), though the values subsequently tended to equalize. The study included alternative treatments (prone position, nitric oxide [NO] or high-dose corticosteroids) that were added in both groups according to the decision of the supervising physician, and which could have partially influenced the results. In 2005, Bollen et al.,31 in a similar study comparing HFOV with pressure-controlled ventilation, but adjusting the TVs, likewise recorded no differences in mortality after 30 days (HFOV 43% versus conventional ventilation 33%; p=0.59). The post hoc study showed that alternative treatment with HFOV could reduce mortality in patients with a poorer oxygenation index at the start of treatment.
In sum, further studies are needed in order to define HFOV as an alternative for improving oxygenation versus the conventional ventilation strategies, and conclusive data referred to survival are also lacking. Application in any case requires specific machinery and centers with experience in using the technique.
Non-ventilatory optionsThe application of simultaneous non-ventilatory therapies may be of great help in improving severe hypoxemia. As alternatives, mention will be made of muscle relaxants, inhalatory or vasoactive drugs, the prone position, and extracorporeal oxygenation therapies or other second-line strategies, such as the administration of corticosteroids.
Muscle relaxantsThe use of relaxants in hypoxemic patients aims to improve patient–ventilator synchronization in situations of deteriorated respiratory mechanics, with the adoption of lung protecting measures. The use of such drugs is subject to controversy, however, since they are classically associated with the development of myopathy on one hand and with a reduction of the benefits of spontaneous breathing according to different models on the other.
Papazian et al. carried out a series of randomized, controlled studies on the benefits of neuromuscular relaxants.32–34 Using the same method, in patients with criteria of ARDS and treated under deep sedation, they compared placebo versus relaxants. In 2004, these investigators analyzed the evolution of oxygenation in the two groups, and recorded sustained and significant improvement in the group treated with relaxants versus the controls. The results were attributed to improved thoracic distensibility and to a decrease in oxygen consumption. In the year 200633, the authors analyzed inflammatory markers in serum and lung samples (bronchoalveolar lavage), and observed buffering of the proinflammatory response, with decreases in the levels of IL-1, IL-6 and IL-8 in the muscle relaxant group versus the controls. In 2010, a multicenter study was carried out34 including 340 patients with PaO2/FiO2<150, and comparing cisatracurium versus placebo. No decrease in mortality was recorded after 90 days in the cisatracurium group versus the controls (31.6 versus 40.7%; p=0.08), though the Cox regression model associated cisatracurium with a protective effect (RR=0.68; 95%CI 0.48–0.98; p=0.04). The post hoc study showed clear benefits in patients with PaO2/FiO2<120. Likewise, a lesser incidence of barotrauma was observed in the cisatracurium group (RR=0.43; 95%CI 0.2–0.9; p=0.03), with a similar incidence of myopathy (64.3 versus 68.5%; p=0.51). Despite the important repercussion of the study, there are doubts regarding its interpretation.35 The muscle relaxants were only used during the first 48h, and the differences in mortality, as reflected by the Kaplan–Meier plots, were observed from day 12 onwards. Survival was greater than expected in both groups, which implied a loss of statistical power–a larger sample (885 patients) being needed to demonstrate differences in mortality. In turn, a greater number of infractions of the lung protecting measures were noted in the control group, which explained the greater incidence of barotrauma and, lastly, relaxation was compared versus deep sedation–a fact that precludes extrapolation of the benefits of spontaneous ventilation.25
Therefore, the utilization of muscle relaxants suggests an improved prognosis. However, doubts remain regarding the interpretation of the intervening physiological mechanisms–without intending to discredit the results obtained. In any case, the guides recommend the use of a train of four systems in order to avoid the associated deleterious effects.
Drug treatmentsA series of drugs, involving different physiological mechanisms, can offer benefits in terms of increased oxygenation.
In the last 20 years inhalatory nitric oxide (NO) has been used due to its pulmonary vasodilator effect, optimizing the ventilation–perfusion ratio and improving oxygenation. However, from the clinical and prognostic perspective, NO has not been as successful as expected and is now little used, except in extreme situations. The review of 5 randomized studies published by Sokol et al.,36 involving 535 patients, revealed temporary improvement in oxygenation, though with no improvement in terms of mortality. Another metaanalysis37 of 12 studies comprising 1237 patients showed no improvement in terms of oxygenation, survival or days without MV. In contrast, an increased risk of acute renal failure was noted. The risk of intoxication due to high blood metahemoglobin levels is only observed with doses above 80ppm of NO, and the therapeutic effects are generally achieved with doses of <20ppm.
An interesting alternative to NO is represented by the inhalatory prostacyclins (epoprostenol, iloprost, treprostinil). These drugs pertain to the group of prostanoids, which are metabolites of arachidonic acid synthesized in the endothelium and have vasodilator properties. The intravenous route is used in the treatment of pulmonary hypertension with right-side heart failure. These drugs have a very short half-life and therefore must be administered on a continuous basis. The resulting metabolic products moreover have a negligible effect. The inhalatory prostacyclins are known to exert an effect in platelet dysfunction, though without clinical relevance. The few existing studies on their use in ARDS reflect improvement of hypoxemia, but not of the patient prognosis.38
Prone decubitusPlacing the patient in prone (or ventral) decubitus is widely used in many Intensive Care Units for individuals with high oxygen demands. Although the literature does not question the improvement in oxygenation with prone decubitus, it is more complicated to demonstrate improvement in patient survival. The benefit of the prone position is based on inversion of the gravitational forces, reducing the pleural pressure in the dorsal regions. This results in improved ventilation of these zones, increased alveolar recruitment, and optimization of the ventilation-perfusion ratio.
The two prospective studies involving the largest number of patients and which attempted to demonstrate improvement in survival were published by Gattinoni et al.39 and Guerin et al.40 In the multicenter study of Gattinoni et al., comprising 304 patients, two groups were compared: one subjected to conventional treatment and the other treated with sessions in prone decubitus (7h a day during 10 days). The mortality rate was the same for prone and supine (dorsal) decubitus after 10 days (21.1% versus 25%; RR=0.84; 95%CI 0.56–1.27), at discharge from the ICU (50.7% versus 48%; RR=1.05; 95%CI 0.84–1.32), and after 6 months (62.5% versus 58.6%; RR=1.06; 95%CI 0.88–1.28). However, oxygenation improved in the prone decubitus group, and no differences were recorded in terms of pressure ulcers, loss of venous accesses or accidental airway withdrawal. The post hoc analysis showed a decrease in mortality in the prone versus the supine position when PaO2/FiO2<88 and Simplified Acute Physiology Score (SAPS)>49 (23.1% versus 47.2%; RR=0.45; 95%CI 0.25–0.95). The criticized aspects of the study were the use of TV >10ml/kg, late inclusion in the prone position, and the few hours of prone decubitus sessions.
In 2004, Guerin et al.40 studied 791 patients using the same methodology as that of Gattinoni (in this case with 8hours a day in the prone position). They likewise observed no differences in mortality between prone and supine decubitus after 28 days (32.4% versus 31.5%; RR=0.97; 95%CI 0.79–1.19; p=0.7) and 90 days (43.3% versus 42.2%; RR=0.98; 95%CI 0.84–1.13; p=0.7), and no differences in the days of MV were recorded (7.8 versus 8.6 days; p=0.9). As in the previously mentioned study, oxygenation improved in the prone position, and a lesser incidence of ventilator associated pneumonia (VAP) was recorded (1.66 versus 2.14/100 patients-day of intubation). In this study an increased incidence of complications was noted in the prone decubitus group, in the form of obstruction of the endotracheal tube, selective intubation, and pressure ulcers. Although in this case TV was respected and the prone position was introduced early, both the sessions and total application time were limited (8h/session during 4 days, on average). The inclusion of patients proved heterogeneous, and there was important patient cross-over from one group to the other–these being factors that could interfere with the conclusions drawn.
In 2005, Mancebo et al.41 conducted a multicenter study with the aim of overcoming the defects of the previous studies. The prone position was introduced early, with 17h per session and an average duration of 10 days, in patients with ALI or ARDS. Of the 136 recruited patients, those in the prone position group presented a lesser FiO2 (p<0.001), a greater PaO2/FiO2 (p<0.001), and lower levels of TV (p<0.01) and PEEP (p<0.048). The mortality rate during admission to the ICU did not differ between the groups (prone position 43% versus supine position 58%; p=0.12). A total of 28 undesired events were recorded in the prone decubitus group; all of them were reversible and did not affect the prognosis. Taccone et al.42 conducted a multicenter study in 2009, with the creation of two subgroups, moderate hypoxemia (PaO2/FiO2 100–200mmHg) and severe hypoxemia (PaO2/FiO2<100mmHg), within the prone decubitus group (18h/session, 8 days) and the supine decubitus group. The difference in mortality rate between the two groups after 28 days and 6 months was not significant (31% versus 32.8%; RR=0.97; 95%CI 0.84–1.13; p=0.7, and 47% versus 52.3%; RR=0.9; 95%CI 0.73–1.11; p=0.33, respectively). The subgroups likewise showed no differences in mortality after 28 days for moderate hypoxemia (25.5% versus 22.5%; RR=1.04; 95%CI 0.89–1.22; p=0.62), though there was a nonsignificant tendency toward lesser mortality in severe hypoxemia with the prone position (37.8% versus 46.1%; RR=0.87; 95%CI 0.66–1.14; p=0.31). The adverse effects were significantly greater in the prone position, with at least one complication per patient (159/168 [94.6%]), compared with the supine position (133/174 [76.4%]).
The joint analysis of the four described studies carried out by Gattinoni et al.43 reflected a 10% decrease in mortality favorable to the prone position in cases of severe hypoxemia, when applied early (in the first 72h) and for a prolonged period of time (>16h/day). In contrast, in the group with moderate hypoxemia, the questionable benefit in terms of mortality versus the risks of spontaneous extubation, accidental disconnections and pressure ulcers worsened the risk–benefit ratio.
Simultaneously, the metaanalysis carried out by Sud et al.44 assessed the importance of the degree of hypoxia and the effect of prone decubitus upon survival. These authors compared 10 studies of great methodological quality and homogeneity, differentiating between moderate hypoxemia (PaO2/FiO2>100) and severe hypoxemia (PaO2/FiO2<100). The prone position significantly improved survival in severe hypoxemia (RR=0.84; 95%CI 0.74–0.96; p=0.01), with the need for 11 patients in the prone position to avoid one death (95%CI 6–50). However, the prone position increased the risk of pressure ulcers (RR=1.29; 95%CI 1.16–1.44), obstruction of the endotracheal tube (RR=1.58; 95%CI 1.24–2.01), and accidental drain withdrawal (RR=3.14; 95%CI 1.02–9.69).
In conclusion, the prone position is an effective rescue strategy for improving oxygenation. A large body of data warrants the possibility of influencing survival in severe cases. The complications in the prone position appear to be more frequent, depending directly upon the duration of the sessions and, probably, on the experience of the supervising team. However, the benefit–risk ratio advises application of the technique in the more seriously ill patients.
Extracorporeal systemsExtracorporeal circuits, such as extracorporeal membrane oxygenation (ECMO), aim to reduce the effect of lung injury induced by MV. In general, this technology has been applied in the treatment of neonatal or pediatric respiratory distress, where its efficacy has been demonstrated45. However, few centers apply such techniques to adult respiratory failure, in view of the questionable results obtained. In 2008, Schuerer et al.45 published a review of the indications of ECMO, based on data from 145 centers throughout the world. Since then a series of inclusion criteria have been established: severe respiratory failure (PaO2/FiO2<100) under MV for less than 7 days, in patients under 65 years age, and the absence of major comorbidities or contraindications for anticoagulation. The results showed a survival rate of >80% in neonatal respiratory failure and of >60–70% in pediatric respiratory failure. In adults, however, survival did not exceed 40%. In the case of heart failure the results were disappointing, regardless of patient age (survival rate 30–40%).
The CESAR study, published by Peek et al.,46 compared conventional treatment of ARDS versus ECMO. A total of 103 hospitals were involved, and 180 patients were recruited and equally distributed between the two arms. The patients randomized to ECMO were transferred to the coordinating hospital. The survival rate after 6 months was greater in the ECMO group (63% versus 47%; RR=0.69; 95%CI 0.05–0.97; p=0.03). There are some methodological objections to this study, however, since adherence to the lung protecting measures was greater in the coordinating center, and other therapies capable of influencing the results were combined, such as HFOV, prone decubitus or nitric oxide.
In 2009, Nehra et al.47 analyzed the results of 81 patients receiving ECMO between 1990 and 2008. The overall survival rate was 53% and, on stratifying the results according to disease condition, was found to be greater in patients with viral or bacterial pneumonia (78% and 53%, respectively) than in trauma or burn patients (33%). Although neonates were not included, the mean age was 23 years (range 2 months–61 years). By age groups, the survival rate was high up to 9 years of age (72%), followed by a decrease to 62% between 30 and 39 years, and a mere 40% in patients >40 years of age. Mortality was greater in cases of multiorgan failure versus respiratory failure only (60% versus 33%).
The Spanish Society of Intensive Care Medicine (SEMICYUC) collaborated in the creation of a registry of patients with ECMO during the A-H1N1 flu epidemic.48 Of the 239 patients registered in 148 ICUs, ECMO was used in only 9 individuals (4%), and on an early basis (4.5 days of MV). Four died during the technique; another died after suspending ECMO as a result of improvement, though followed by subsequent complications; and the remaining four patients survived (44%). The main bias in the study was the small number of patients, though the international surveys have published similar results in terms of survival and complications.
In conclusion, ECMO is difficult to introduce, expensive, and requires an important infrastructure (third-level hospital centers). In addition, its benefits in terms of patient survival are not clearly better than those of other techniques which are more accessible in any center.
Corticosteroid treatmentCorticosteroids continue to produce controversy because of their potential adverse effects in terms of muscle atrophy and/or an increased frequency of infections.
Meduri et al.49 started to treat ARDS in the first 72h and during 28 days using methylprednisolone. On day 7 they observed increased extubation success versus the control group (53.9% versus 25%; p=0.01), fewer days of MV, a shorter stay in the ICU, lesser mortality (20.6% versus 42.9%; p=0.03), and a better PaO2/FiO2 ratio (256 versus 179mmHg; p=0.006). The authors attributed these results to attenuation of the inflammatory response induced by the corticosteroid treatment. The study of the ARDS Network50 compared methylprednisolone versus placebo from day 7 of distress, and recorded advantages as well as disadvantages. Improvement was recorded in terms of oxygenation, days without MV, and the need for vasoactive drugs. In contrast, muscle atrophy increased, and survival did not improve after either 60 or 180 days; indeed, survival even decreased among those patients in which treatment was started from 14 days after the onset of distress. Lastly, the metaanalysis published by Tang et al.51 analyzed 9 studies in which corticosteroids were used in application to distress. The mortality risk was found to be lower when administering corticosteroids (RR=0.62; 95%CI 0.43–0.91; p=0.01). Likewise, the duration of stay in the ICU, the days of MV, and the number of infections or multiorgan failures were all lower in the corticosteroid group, and no increased incidence of myopathy was observed.
The data suggest that if the use of corticosteroids is decided, benefits are obtained only if treatment is started early; contrarily, the results may prove negative.
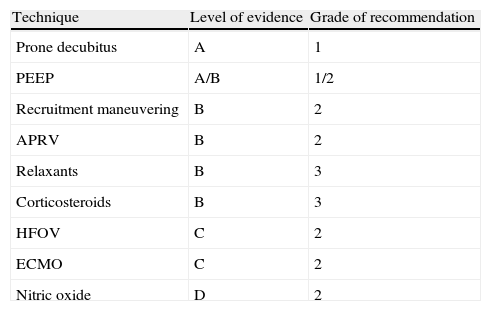
ConclusionRefractory hypoxemia represents the most advanced stage of ARDS, where the life-threatening circumstances suggest the need for aggressive rescue interventions. In both ventilation treatment, as the cornerstone of patient management, and in the non-ventilatory interventions, the fundamental aim is to improve oxygenation and the ventilation–perfusion balance, increasing the gas exchange surface. Table 1 shows the grades of recommendation for each of the techniques, on the basis of the data obtained from the analyzed studies.
GRADE scale for the estimation of recommendations.
| Technique | Level of evidence | Grade of recommendation |
| Prone decubitus | A | 1 |
| PEEP | A/B | 1/2 |
| Recruitment maneuvering | B | 2 |
| APRV | B | 2 |
| Relaxants | B | 3 |
| Corticosteroids | B | 3 |
| HFOV | C | 2 |
| ECMO | C | 2 |
| Nitric oxide | D | 2 |
Level of evidence: A, high; B, moderate; C, low; D, very low.
Grade of recommendation: 1, most specialists would choose this option; 2, many specialists would choose this option, but a substantial proportion would not; 3, recommended on the basis of consensus, though individual criterion prevails.
In conclusion, in critical patients with refractory hypoxemia and life-threatening conditions, all options should be considered, relying in all cases on the clinical experience of the center and the availability of resources, and seeking to avoid further patient harm as a permanent guiding principle.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Delgado Martín M, Fernández Fernández R. Estrategias frente a la hipoxemia refractaria en el síndrome de dificultad respiratoria del adulto. Med Intensiva. 2013;37:423–430.
Two important studies have been presented while this article was awaiting publication. The first, presented by Guerin et al. (ESICM Congress 2012), demonstrates very significant improvement in severe ARDS mortality with prone decubitus. The second, published by Ferguson et al. (NEJM, January 2013, 22 [Epub ahead of print]), reports no improvement in ARDS survival with high-frequency oscillatory ventilation.